- Home
- Advocacy
- Latest News and Practice Data
- February 18, 2020
Read the Latest Issue of Advocacy Update
February 18, 2020
In This Issue:
- CAP-Backed Legislation on Surprise Bills Advances Through Committee
- Texas Pathologists, CAP Oppose Regulation that Increases Administrative Burdens on Patients
- Dr. Kozel: How You Can Champion Health Policy with PathPAC
- 2019 Practice Characteristics Survey Insights: Pathology Practice Setting
- CDC Updates Laboratory Biosafety Guidelines for Coronavirus
- Test Your Advocacy Knowledge with the February News Quiz
CAP-Backed Legislation on Surprise Bills Advances Through Committee
On February 12, the House Ways & Means Committee overwhelmingly voted to support the CAP-backed Consumer Protections Against Surprise Medical Bills Act of 2020 to resolve billing disputes between insurers and physicians. This legislation includes several positions advocated for by the CAP and its members, including an open mediation, or independent dispute resolution process, without an arbitration threshold. Further, the Ways & Means legislation does not have a benchmark payment and includes several patient protections supported by the CAP.
The CAP remains engaged with the committee and House leaders to ensure pathologists’ concerns on this issue are fully addressed in the bill. Still, the Ways & Means legislation represents the best outcome available of all the proposed solutions on surprise medical bills considered by congressional committees, so far. The Ways & Means bill was voted on and approved by committee members with overwhelming support.
The Ways & Means Committee is the latest panel with jurisdiction over surprise bills to mark-up legislation. Other legislative proposals introduced and debated by the Senate Health, Education, Labor, & Pensions Committee, House Energy and Commerce Committee, and the House Education and Labor Committee have favored insurance companies and have fallen well short of taking a balanced approach to eliminating surprise medical bills. Their proposals rely on rate-setting based on median in-network rates that remove any incentive for insurers to negotiate in good faith and may lead to even narrower insurer networks of physicians. The CAP strongly opposes these other proposals.
Continue to Urge Your Representatives to Act on Surprise Bills
There is still time to go to the CAP’s action center to tell your representatives in Congress to support our positions on surprise medical bill legislation. Enter your email and ZIP code to login and select the legislative alert on surprise billing. In just a few clicks, you can easily communicate with your elected officials.
Texas Pathologists, CAP Oppose Regulation that Increases Administrative Burdens on Patients
The Texas Society of Pathologists (TSP), the Texas Medical Association (TMA), and the CAP opposed the latest effort from the state’s Department of Insurance to increase administrative burdens on patients in the latest state regulation related to surprise medical bills. The CAP is committed to reducing the administrative and regulatory burdens hindering patients from receiving critical life-saving diagnoses and care.
The proposed regulation will require out-of-network pathologists to provide patients with a waiver outlining out-of-network charges, including a highly detailed written estimate of services, for patient consent, and then require patients and providers to wait between 10 and 15 days before any pathology services are performed.
The TSP, TMA, and CAP all agree the regulation contradicts the intent of original Texas Senate Bill 1264, which prevented patients’ health insurance from paying excessive medical bills for out-of-network services. Instead, the insurers and physicians would negotiate how to pay the non-patient share of the costs.
In a February 10 letter, James S. Malter, MD, FCAP, President of the Texas Society of Pathologists, urged the Department of Insurance to enforce SB 1264 fully. The law, “allows for an exception to the prohibition on balance billing if the patient is informed and can make an educated and rational determination that utilizing that exception would be in the patient’s best interest. The members of TSP are greatly concerned that the Texas Department of Insurance has fashioned an emergency regulation and is preparing to implement a rule creating a process to provide for that exception that is inconsistent with the statutory intent of Senate Bill 1264,” the letter said.
The new proposed regulation would administratively expand the scope of what the legislation SB 1264 was intended to do. Dr. Malter further explained that the regulation “has no statutory basis in Texas and impedes health care delivery (for at least, potentially, 15 days, if not more when the amount of time required for a dubious written estimate is included) for those patients who wish to select out-of-network pathology/laboratory services.” This would increase the administrative burden, not only on pathologists but on patients as well, hindering quality patient care.
The TSP and the CAP will continue monitoring the issue and provide updates.
Dr. Kozel: How You Can Champion Health Policy with PathPAC
Periodically, CAP Advocacy features one of the many CAP members who are champions for pathology through their advocacy at the federal or state level through our grassroots and PathPAC programs. If you would like to get involved, you can join PathNET, contribute to PathPAC, or join your state pathology society.

Recently Advocacy Update caught up with Jessica Kozel, MD, FCAP, who is an associate pathologist in Saint Luke’s Hospital System in Kansas City, Missouri. Dr. Kozel has donated to the PathPAC as she knows it can shape health policy for pathologists.
What drove you to get involved in advocacy? Several of my mentors, including Leilani Valdes, MD, FCAP; Kalisha Hill, MD, FCAP; CAP Secretary-Treasurer Rick Gomez, MD, FCAP; and Sam Caughron, MD, FCAP; among others, have always stressed how important advocacy is for our profession. Now that I’ve got five years of practice under my belt, I feel like it is a perfect time to help give back to our profession through advocacy.
Do you have a favorite memory or experience that stands out in your advocacy work? My first Hill Day, in association with the CAP Policy Meeting, was such a fantastic experience. It made me proud of my country, the democratic process, and my profession to make our voices heard. I had a great mentor with Dr. Gomez, who showed the way so that helped calm my nerves, as I’m an introvert pathologist, so this was out of my comfort zone!!
What advice would you give to your colleagues in order to be effective advocates? Do what you can.
If you can’t or don’t feel comfortable advocating in DC, give to the PathPAC or communicate with your legislators on a grassroots level. We need people advocating for pathology on all fronts, both in their home states and in DC.
If you have an interest in advocating personally, sign up for the Pathologist Leadership Summit. The speakers and more experienced CAP members will guide you along the way.
If you can’t go to the Pathologist Leadership Summit and don’t know how or what to do to get involved, contact one of the Federal and State Affairs Committee members. We are always looking for enthusiastic pathologists to work with us!!
Have you ever done a laboratory tour? If so, are there any tips you would like to share with your colleagues? No. But I would love to do one in the future.
Why did you choose to donate to the PathPAC? Supporting advocacy for our specialty, both monetarily and through volunteer service, is a professionalism issue. If you want to be a professional, you must make legislators know how and why your work is so important to the US and their constituents (as known as “our patients”).
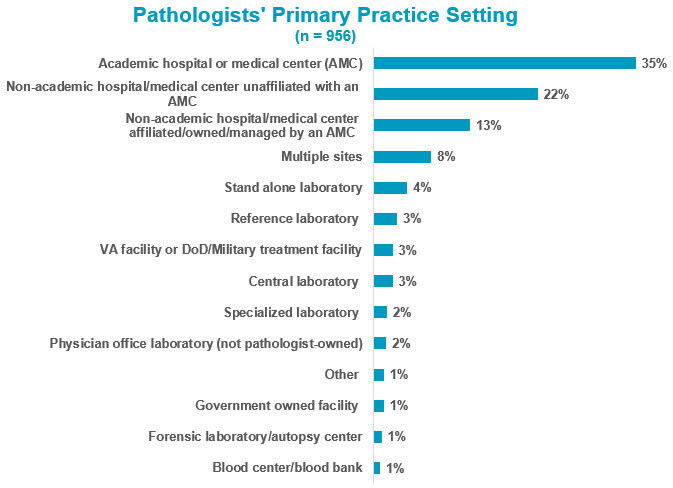
2019 Practice Characteristics Survey Insights: Pathology Practice Setting
The 2019 Practice Characteristics Survey provided empirical support for setting advocacy priorities to inform lawmakers and regulators of the essential issues facing pathologists today.
This installment of insights from the 2019 Practice Characteristics Survey highlights findings related to pathologists’ practice setting.
According to the 2019 Practice Characteristics Survey, the number of pathologists working in academic and non-academic settings is roughly equivalent. About 35% of respondents indicated their primary practice setting was an academic hospital or medical center. The non-academic responses were categorized by whether or not their hospital was affiliated with, owned by, or managed by an academic medical center. Still, collectively 35% of respondents indicated they primarily practiced in a non-academic hospital setting.
About 91% of the respondents spent the vast majority of their time—at least 75%—working in a single type of setting. Nearly 8% worked in multiple types of settings, and just over 1% do work that is not dependent on a specific location (eg, locum tenens, consulting).
CDC Updates Laboratory Biosafety Guidelines for Coronavirus
Please note that the Centers for Disease Control and Prevention (CDC) updated its Interim Laboratory Biosafety Guidelines for Handling and Processing Specimens Associated with 2019 Novel Coronavirus (2019-nCoV) on February 10, 2020. The CAP will work with the CDC to monitor the outbreak and laboratory capacity to provide testing. The CAP will provide further updates as new information becomes available.
The latest revisions to the guidelines include:
- The term “certified” was added to clarify that a certified Class II Biological Safety Cabinet (BSC) should be used for any laboratory procedure with the potential to generate aerosols or droplets.
- Additional guidance was provided for any laboratory procedures that are performed outside of a BSC. For any procedures performed outside of a BSC, eye and face protection (eg, goggles, mask, face shield) or other physical barriers (eg, splash shield) should be used to minimize the risk of exposure to laboratory staff.
- Additional guidance was provided on the selection of the appropriate disinfectants to be used to decontaminate laboratory work surfaces and equipment. Use EPA-registered hospital disinfectants with label claims that are effective against other respiratory pathogens, such as seasonal influenza and other human coronaviruses.
- Additional guidance was provided for handling 2019-nCoV laboratory waste. For 2019-nCoV laboratory waste, follow standard procedures associated with other respiratory pathogens, such as seasonal influenza and other human coronaviruses.
- The need for both site- and activity-specific risk assessments was added to determine if additional laboratory biosafety control measures are necessary.
The CDC has received numerous inquiries for guidance on the packing, shipping, and transport of specimens from suspected and confirmed patients infected with 2019-nCoV. At this time, specimens associated with suspected and confirmed patients should be packed, shipped, and transported as UN 3373 Biological Substance, Category B.
For additional guidance on packing instructions (IATA Packing Instructions 650) and requirements for shipping and transport, refer to the current edition of the International Air Transport Association (IATA) Dangerous Goods Regulation.
For additional information related to the Interim Laboratory Biosafety Guidelines for Handling and Processing Specimens Associated with 2019-nCoV, refer to the CDC 2019 Novel Coronavirus Laboratory Biosafety website.
Test Your Advocacy Knowledge with the February News Quiz
Take the Advocacy News Quiz and see how you stack up against your fellow CAP members. Try and share your results on social media. Take the February news quiz today.