- Home
- Laboratory Improvement
- Accreditation
- Biorepository Accreditation Program
High quality biospecimens are needed to improve patient outcomes. As a result of unprecedented research in precision medicine, demand for fit for purpose biospecimens is growing rapidly. According to a US survey of National Cancer Institute (NCI) funded cancer researchers, lack of quality biospecimens resulted in 60% of researchers questioning their findings and 81% limiting the scope of their work.1
Excellence and quality measures in biorepositories are interrelated concerns that have a direct bearing on research that can help advance health care. Pioneered in 2012, the CAP Biorepository Accreditation Program was the first accreditation program designed to improve the quality and consistency of biorepositories. Drawing on best practices from ISBER, NCI, OECD, CMS and the Laboratory Accreditation Program, the goal of the program is to provide requirements for standardization of processes that will result in high-quality human specimens and genetic materials that can be used to support research. Recently, CAP physician members contributed to the ISO technical committee that developed the ISO 20387 standard for biobanking.
Frequently Asked Questions
A biorepository is defined as an entity that receives, stores, processes, and/or disseminates biospecimens, their derivatives, and relevant data, as needed. For purposes of CAP accreditation, a biorepository encompasses the physical location as well as the full range of activities associated with its operation. Activities that may be performed by biorepository personnel: patient consent, specimen collection, specimen processing, quality control, quality assurance processes such as sample characterization testing for suitability of the sample for downstream use, specimen storage, data management, sample release and tracking.
Our goal is to improve and standardize quality and consistency in collecting, processing, storing, distributing, and computerizing information for biospecimens while ensuring the quality of human specimens (eg, serum, urine, blood, and tissue) and genetic material (eg, RNA and DNA). The BAP checklists are reviewed annually and compared with applicable requirements from other organizations such as: ISBER Best Practices; NCI Best Practices; ISO 20387.
No. The program is voluntary. However, some biorepositories may want to demonstrate their CLIA-equivalence with an actual Certificate of Accreditation from CLIA. CLIA regulations apply to facilities (laboratories) that perform at least one test that impacts a human being. Because biorepositories under BAP may include the storage of extracted nucleic acid as a sample type, this started the process of obtaining CLIA acceptance of the BAP requirements. In 2019, the BAP checklists were submitted to CMS and accepted.
Inspection and accreditation will drive consistency in achieving compliance with multiple processes within a biorepository. There are many best practices within the industry, but wide variability in their application. Accreditation will ensure a more level playing field. Specifically, biorepository accreditation from the CAP helps to ensure:
- Appropriate ethical and legal frameworks for use of biospecimens in IRB-approved research
- Well-controlled pre-analytic variables for optimal scientific investigation or biomarker development
- Robust chain of custody tracking, reduced risk of misidentification
- Appropriate storage conditions and temperature monitoring
- Best practice policies and procedures for sample release
- Histologic quality assurance for starting tumor content in samples homogenized for downstream assays
- Confidence in the long-term quality of biospecimens
- Differentiation for research/grant funding opportunities
Eligibility Requirements
The program is only applicable to those facilities that receive, store, process, and/or disseminate biospecimens, their derivatives, and relevant data for research purposes. The program is not applicable to tissues being stored for transplant purposes however, the CAP Laboratory Accreditation Program (LAP) does inspect the storage of transplant tissues when they are under the purview of the laboratory director. Retained clinical samples not intended for research testing are not covered under BAP.
Program Details
- On-site inspections occur every two years using our CAP Accreditation Checklists to assess compliance with program requirements.
- Participating biorepositories can access the checklists through e-LAB Solutions Suite; and non-accredited biorepositories, may purchase the checklist.
- Peer-based inspection model uses teams of practicing professionals qualified through a CAP inspector training program.
2019 Biorepository Checklist Requirements
Diagnostic patient testing must be performed within a CLIA licensed laboratory. In 2019, CMS approved our biorepository checklist requirements as being consistent with CLIA regulations. Alignment of the checklists from the CAP’s Biorepository Accreditation Program and Laboratory Accreditation program help ensure:
- Confidence in specimen provenance- a clinical laboratory director may accept specimens for testing from a BAP accredited repository because of formal CLIA-approved requirements in specimen collection integrity.
- Confidence in pre-analytic variable tracking and control for samples used in drug trials with associated biomarker development.
- Improved alignment of accreditation preparation and inspection processes for repositories affiliated with CAP-accredited laboratories
The Inspection and Accreditation Process
The inspection program is based on a peer-inspection model. The inspectors
may be pathologists, PhDs, or managers of biorepositories (typically with a
medical technology, biomedical, or nursing background). Most critical is their
current experience in an active biorepository. Inspectors will be qualified
through a CAP training program. The CAP staff inspectors may supplement peer
inspectors to ensure each inspection’s timely execution and quality. One to two
inspectors will inspect most biorepositories.
Peer inspector(s) perform on-site inspection
using CAP accreditation checklists to provide a comprehensive and up-to-date
blueprint of quality practices enabling biorepositories to improve their
operations and ensure quality. The post-inspection desk assessment offers a
remote review of a biorepository’s quality management plan, specified
procedures (related to key accreditation requirements), and select quality and
process statistics. It will assist biorepositories in strengthening their
procedures through the identification of areas that need improvement.
Inspection and Accreditation FAQs
A biorepository interested in the program but not confident in its “readiness” may purchase a set of the checklist requirements and perform a self-inspection. The CAP also has a CAP Accreditation Readiness Assessment (CARA®) that provides a high-level evaluation of the biorepository’s processes using an educational approach.
Yes, they can be purchased by completing the CAP checklist order form.
Any biorepository that purchases the checklists and applies for accreditation within 6 months will not be required to pay the accreditation application fee, because the checklists were purchased in preparation for a CAP inspection.
Complete a request for application form on cap.org which includes a one-time, non-refundable application fee. Upon receiving the request, the CAP will send an application and provide access to our customer portal, e-LAB Solutions Suite to download checklists and other accreditation resources.
Once the CAP receives the completed application, an initial inspection readiness conference call will be held with CAP staff members and then CAP will assign an inspection team leader and a team will be assembled. The team will conduct an inspection. If deficiencies are cited, the facility must provide documentation in support of the correction or plan of correction for each deficiency cited. The CAP staff and pathologists who actively lead our accreditation programs will perform a multi-tiered review of the findings to render an accreditation decision.
No, enrollment in proficiency testing is not required under BAP. However, some biorepositories may choose to enroll in proficiency testing as a mechanism for the periodic assessment of the quality of the stored specimens. The CAP Surveys and Anatomic Pathology Education Programs may include products that are applicable to biorepositories.
Per ISO 20387 7.8.2.9: The biobank shall use approaches to provide objective evidence to demonstrate the comparability of biological material quality (the processing or testing output), where such approaches are available and appropriate. Such approaches include external quality assessment (EQC) programs proficiency testing programs, approaches, including the use of:
- Certified reference materials, where available, produced by a reference material producer fulfilling the requirements of ISO 17034;
- Samples previously examined;
- Samples previously shared with other biobanks
- Control Materials that are testing regularly in EQA programs
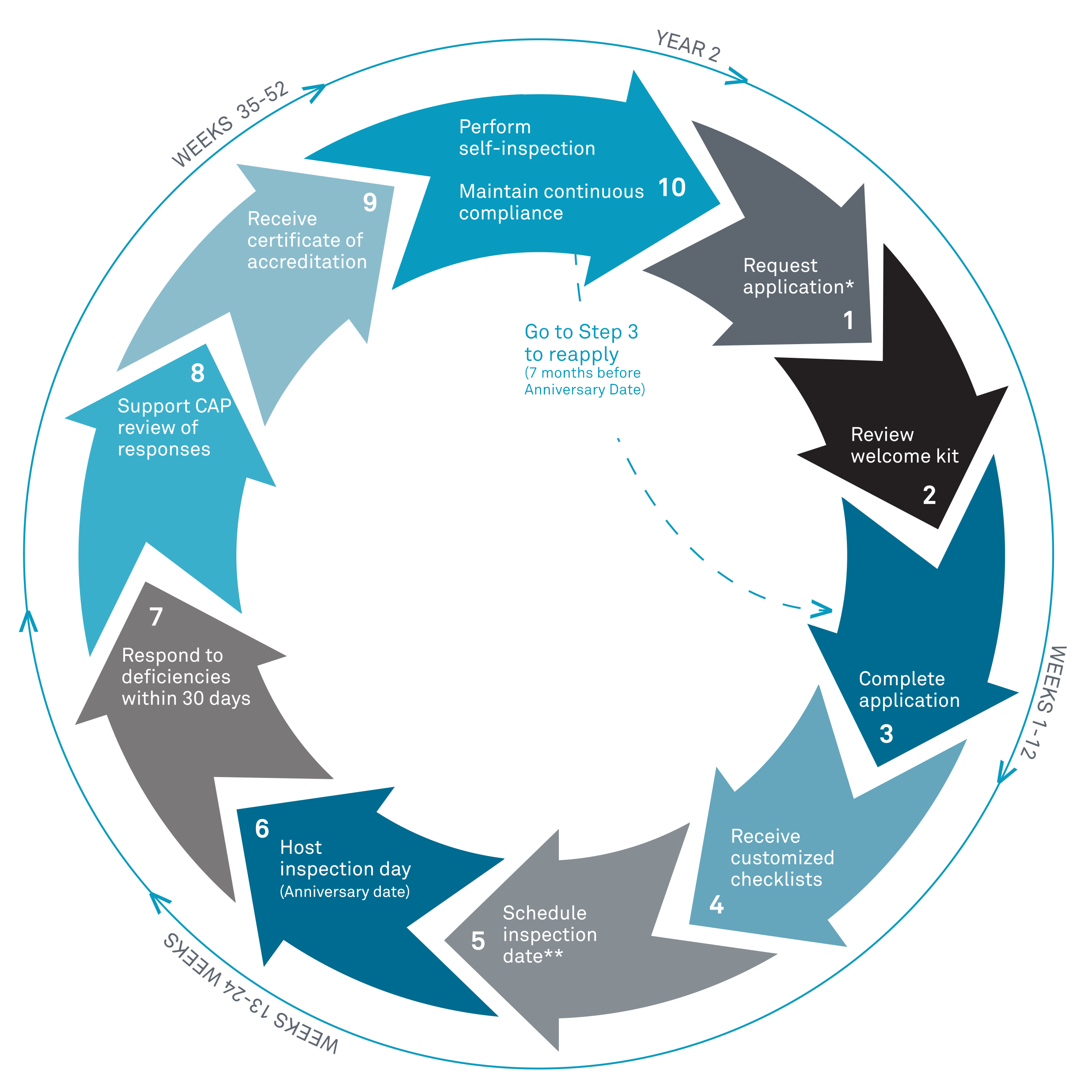
The Accreditation Cycle
The CAP had changed the on-site inspection cycle for
biorepositories from inspection every three years to every two years with an
interim self-inspection required in the off year. The two-year accreditation
cycle is consistent with the CAP’s other accreditation programs.
Find a CAP-Accredited Biorepository
Use our database to search for CAP-accredited biorepositories.
1. Assessing the need for a standardized cancer HUman Biobank (caHUB): findings from a national survey with cancer researchers. Massett HA1, Atkinson NL, Weber D, Myles R, Ryan C, Grady M, Compton C.