- Home
- Advocacy
- Latest News and Practice Data
- FDA, International Organizations Issue Artificial Intelligence Guiding Principles
On June 13, the FDA, Health Canada, and the United Kingdom’s Medicines and Healthcare products Regulatory Agency jointly issued the Transparency for Machine Learning-Enabled Medical Devices: Guiding Principles. In 2021, the groups jointly identified ten guiding principles for good machine learning practice. The guiding principles released June 13 build upon the 2021 principles. These principles support the development of safe, effective, and high-quality artificial intelligence/machine learning technologies that can be learned from real-world use and, in some cases, improve device performance.
They further identified guiding principles for transparency for machine learning-enabled medical devices which build upon the 2021 principles. Effective transparency is as follows:
- ensures that information that could impact risks and patient outcomes is communicated.
- considers the information that the intended user or audience needs and the context in which it's used.
- uses the best media, timing, and strategies for successful communication,
- relies on a holistic understanding of users, environments, and workflows.
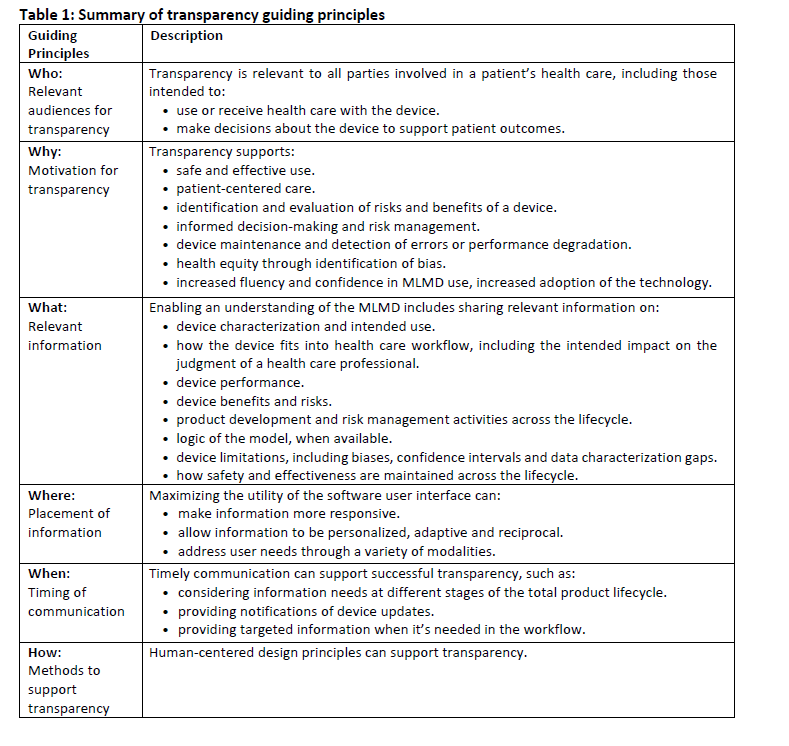
These guiding principles are intended as considerations when adopting and advancing good transparency practices.
In prior advocacy to the FDA and the ONC, the CAP has supported transparency in AI/ML devices, noting that transparency must be required in any regulatory framework, which should mandate that developers implement an open system that describes updates and modifications as they occur to patients and clinicians.