- Home
- Member Resources
- Pathology Case Challenge
- Respiratory Infection
Clinical Summary
A 55-year-old HIV-positive man presents with weakness and a dry cough for two weeks at your clinic in Texas. You gather a quick history on the patient and learn he visited his sister in Ohio several months previously. The patient mentioned that his sister has several chicken coops that he tended during his visit. You screen him for SARS-CoV-2, and he tests positive for COVID-19.
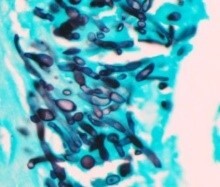
The patient was found to exhibit prominent cervical lymph nodes. Small yeast forms were seen in fine-needle aspiration of lymph nodes without evidence of malignancy (Image 1).
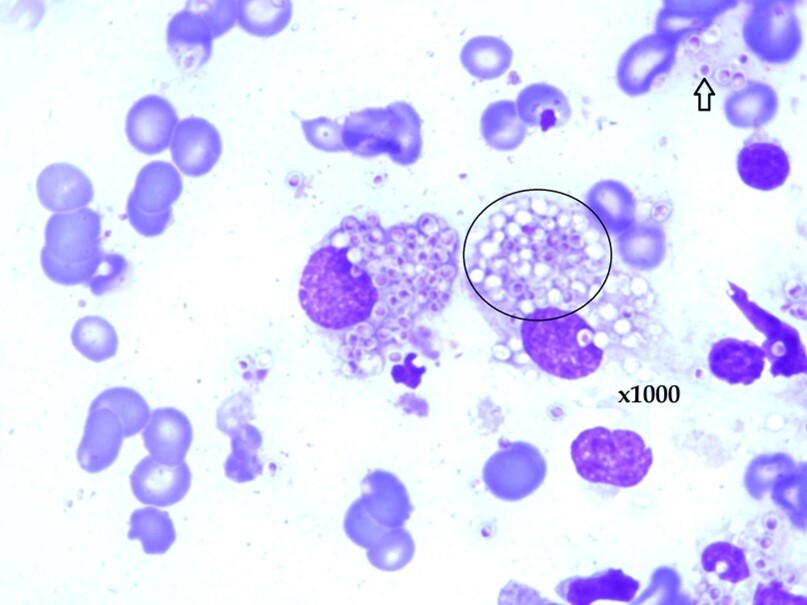
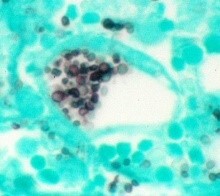
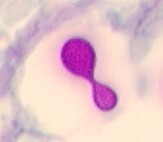
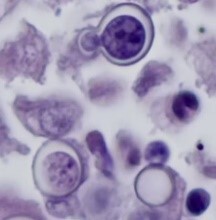
Three months later, the patient appeared with abdominal pain and recurrent diarrhea that has increased over the past several weeks. The patient was able to pass stool without blood. Small bowel biopsy was conducted and results are presented (Image 2, Image 3, and Image 4).
Master List of Diagnoses
- Blastomyces dermatitidis
- Sporothrix schenckii
- Histoplasma capsulatum
- Leishmania spp.
- Trypanosoma spp.
- Cryptococcus neoformans
- Talaromyces marneffei
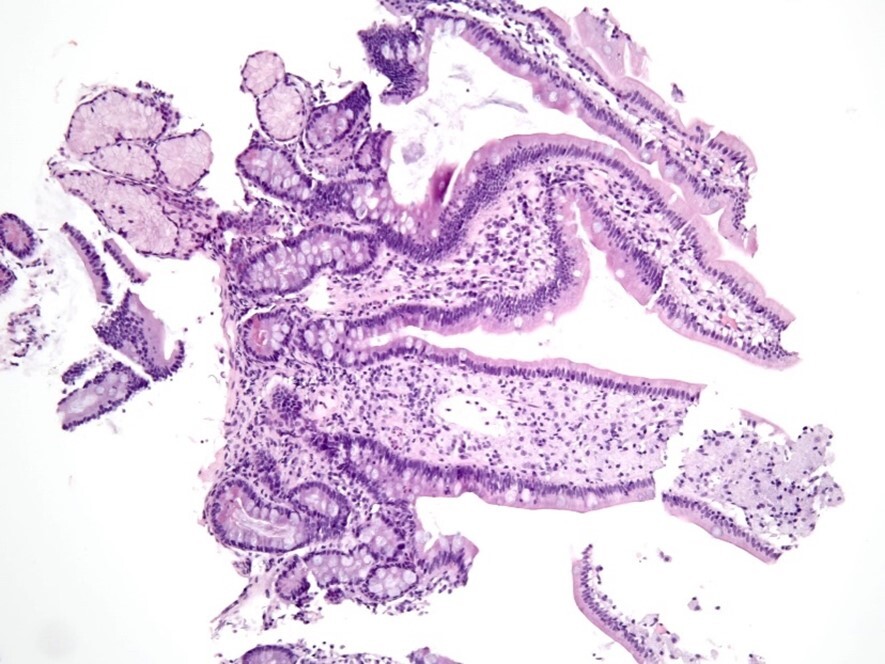
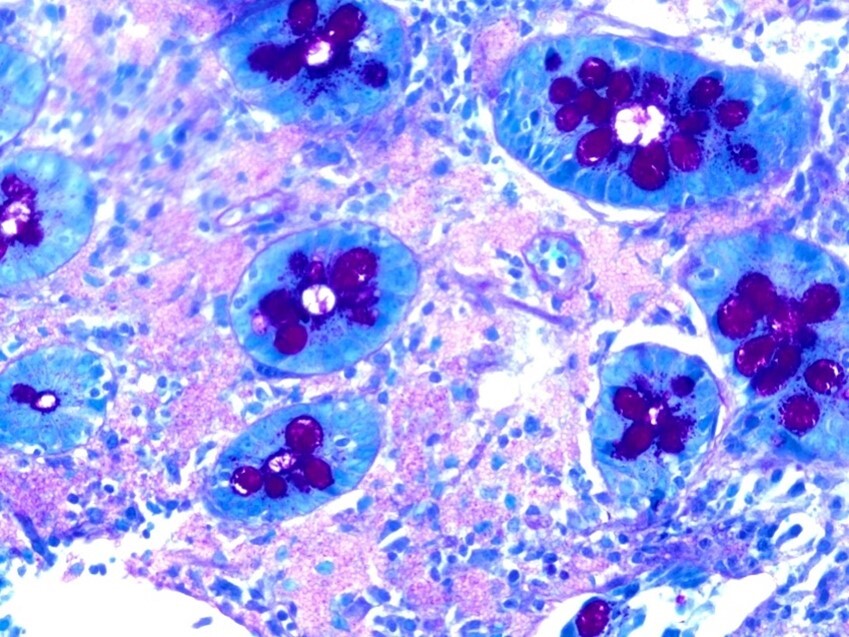
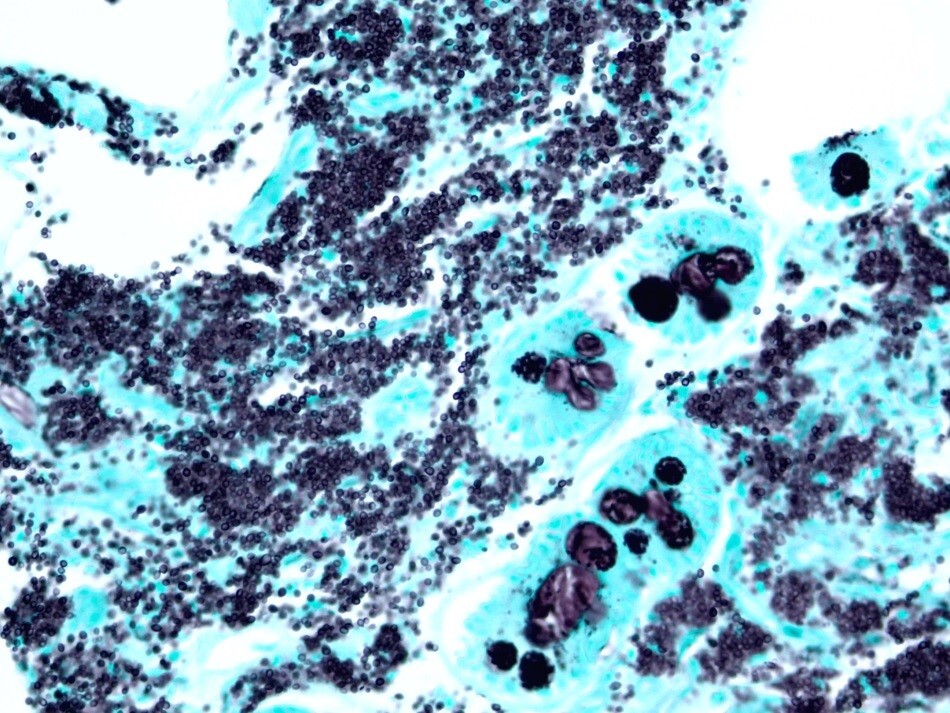
Archive Case and Diagnosis
This material was originally released as 2021 CPIP-L Case 12: Microbiology.
Criteria for Diagnosis and Comments
Histoplasmosis is endemic along the Ohio and Mississippi river valleys in the United States in which infection is seen in areas characterized by soil contaminated with bird or bat guano such as chicken coops, caves, or turned soil.1 After histoplasmin skin testing established the original endemic zones in the 1940s to 1950s, histoplasmosis outbreaks have been located in 26 states, including California and Arizona and the territory of Puerto Rico, considerably outside of the Mississippi and Ohio river valleys.2 Ease of travel of infected patients from within to outside of endemic areas and the presence of increased numbers of immunosuppressed patients have combined to increase the presence of histoplasmosis in non-endemic areas of the United States.
Fungal diseases, including histoplasmosis, continue to produce new respiratory infections during outbreaks or pandemics of other respiratory infections (eg, COVID-19). Providers need to consider fungal respiratory infections in appropriate settings, particularly when COVID-19 testing is negative. Histoplasmosis can uncommonly manifest as acute respiratory distress syndrome in profoundly immunosuppressed patients, a presentation that could be confused with SARS in COVID-19.3 Dual infections of histoplasmosis and COVID-19 have been noted in several patients.4
The finding of small yeast in fine-needle aspiration of lymph nodes suggests a variety of yeast and yeast-like organisms as well as yeast mimics. These include:
- Blastomyces dermatitidis
- Sporothrix schenckii
- Histoplasma capsulatum
- Leishmania spp.
- Trypanosoma spp.
- Cryptococcus neoformans
- Talaromyces marneffei
Table 1: Yeast types and characteristics.
Organism |
Ecology |
Disease |
Image |
Candida spp. Key histologic features:
|
Normal inhabitant of human skin, gastrointestinal tract, and vagina |
Hematogenously disseminated infections and localized infections including thrush and vaginitis |
|
H. capsulatum Key histologic features:
|
Organism is found in soil enriched with bird or bat guano primarily but not exclusively in the Ohio/Mississippi river valleys in the United States; humans become infected by inhalation of conidia |
Transitory influenza-like illness, acute pneumonia, chronic pulmonary infection, disseminated disease |
|
Cryptococcus neoformans/gattii Key histologic features:
|
C. neoformans is found worldwide in association with pigeon guano, components of which are inhaled to produce infection; C. gattii lives on trees, in tree hollows, and in the soil around trees in tropical and subtropical areas of the world and also in mainland British Columbia, Vancouver Island, the U.S. Pacific Northwest |
Pneumonia, disseminated disease and meningitis in immunocompromised patients; It has been suggested that C. gattii may have a propensity to infect immunocompetent individuals |
|
Blastomyces dermatitidis Key histologic features:
|
B. dermatitidis lives in decaying wood and leaves but the understanding of its ecological niche is incomplete; Endemic areas of the United States and Canada include the Ohio and Mississippi river valleys and the Great Lakes |
Acute and chronic pulmonary infections, skin infections, and disseminated disease |
|
Sporothrix schenckii Key histologic features:
|
S. schenckii is found in soil and decaying plant material worldwide |
Produces lymphocutaneous sporotrichosis of the forearm after a puncture wound |
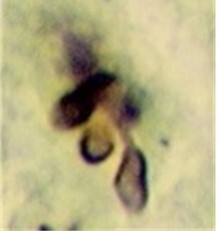
|
Talaromyces marneffei (formerly Penicillium marneffei) Key histologic features:
|
Talaromyces marneffei is found in Southeast Asia, Southern China, and Eastern India. Infection is through inhalation of conidia |
Human disease is rare and often occurs in immunocompromised patients particularly HIV/AIDS |
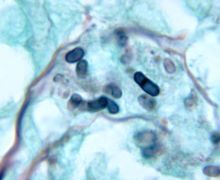
|
Reference: Love GL, Ribes JA. Color Atlas of Mycology. CAP Press; 2018. ISBN:978-1-941096-42-0 |
|||
The PAS stain is an excellent fungal stain but may stain the large numbers of yeast in disseminated histoplasmosis as a bubbly, pink mass which potentially could be passed over at lower magnifications. GMS stains individual H. capsulatum forms that are easier to recognize. Other yeast that may be similar in size to H. capsulatum include Cryptococcus neoformans/gattii, and Sporothrix schenckii. C. neoformans may be seen in a range of sizes from 2 to 8 µm in diameter. When present in the smaller range of 2 µm, C. neoformans could be morphologically similar to H. capsulatum and both yeasts may be intracellular. Mucicarmine and Fontana-Masson stains stain C. neoformans with negative staining of H. capsulatum. Sporothrix schenckii is a pleomorphic yeast usually not seen in large numbers. Talaromyces marneffei is a thermodimorphic yeast-like fungus seen in Thailand and countries in the Asian rim. T. marneffei does not bud like a true yeast but rather breaks apart by fission. At lower magnifications, T. marneffei could look very similar to H. capsulatum and the GMS stain would be helpful for discrimination between the two. Blastomyces dermititidis is a larger yeast (about 8 micrometers in diameter) with a thick cell wall and broad-based budding.
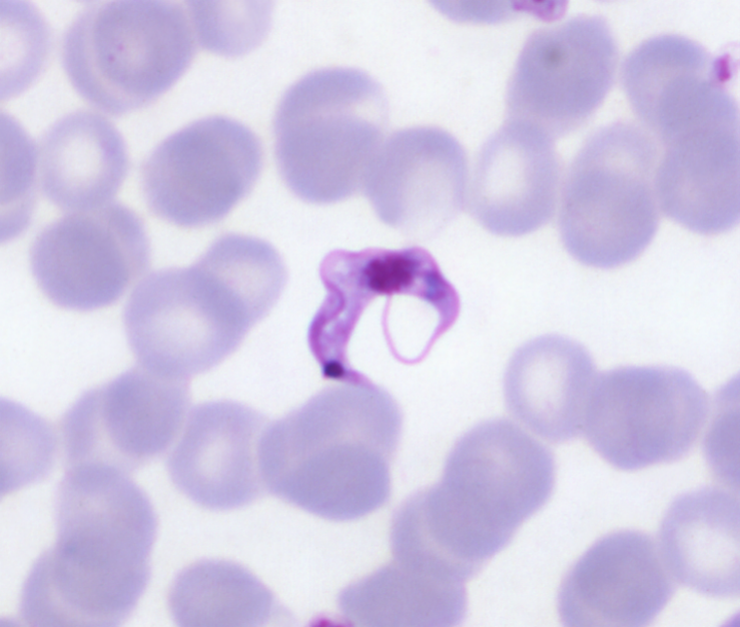
A strikingly similar appearance in aspirated material may be seen with amastigote forms in leishmaniasis (Image 5) and trypanosomiasis (Image 6). These diseases can be distinguished by morphology and clinical history.
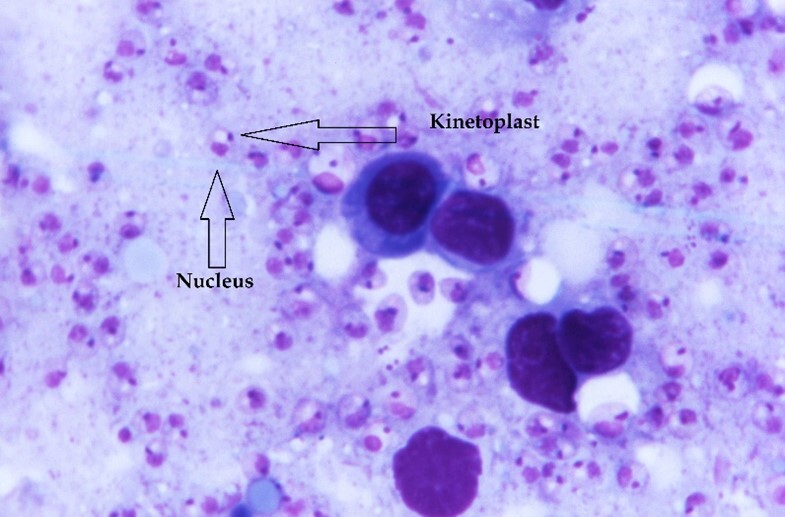
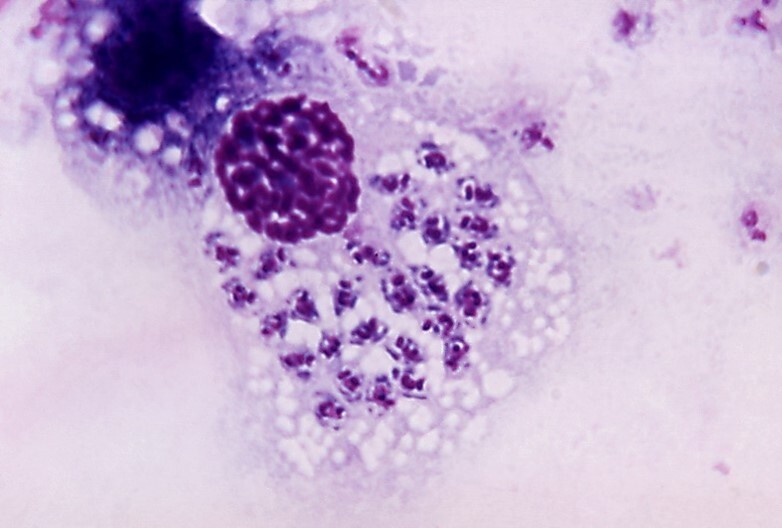
Trypanosomes and Leishmania are taxonomically related kinetoplasmid protozoan parasites that have similar structural and biochemical features. These include a single mitochondrion with a discrete structured DNA body: the kinetoplast, the presence of which helps distinguish Leishmania and Trypanosoma amastigotes from yeast of H. capsulatum which lack the organelle. H. capsulatum also possesses a cell wall that Leishmania and Trypanosoma amastigotes lack. The cell wall may be easier to appreciate by focusing through the tissue plane and back. History and clinical presentation also help separate the diseases of histoplasmosis, leishmaniasis, and trypanosomiasis.
Leishmaniasis differs from histoplasmosis in several clinical respects. The endemic area for histoplasmosis is along the Ohio and Mississippi river valleys in the United States whereas primary infections of Leishmania rarely occur in the United States. Most infections in the Western Hemisphere occur in parts of Mexico, Central America, and South America with only few cases reported in Texas and Oklahoma.5
As compared to inhalation of organisms in the case of histoplasmosis, primary infection of Leishmania is through the bite of a sandfly. The skin lesion usually remains localized only rarely spreading over the skin into multiple, disseminated or diffuse lesions. Cutaneous lesions can persist for years and may be associated with enlargement of regional lymph nodes or lymphangitis which, if on the forearm, may be confused with lymphangitic spread of Sporothrix schenckii. Mucosal and visceral leishmaniasis also occur. Mucosal leishmaniasis develops from dissemination of organisms from cutaneous lesions and, over years, may produce destructive nasal ulcers. Visceral leishmaniasis is prone to develop in immunocompromised patients, particularly those with HIV/AIDS. Hepatosplenomegaly with a very prominent spleen as well as lymphadenopathy may be produced. Some individuals with visceral leishmaniasis may develop gastrointestinal lesions. A Gomori methenamine silver (GMS) stain would be negative for organisms in these cases with nonspecific positive staining only seen in mucus in intestinal goblet cells. In a note of caution, splenic aspiration may produce life-threatening hemorrhage in the case of visceral leishmaniasis. Bone marrow aspiration is a better means to obtain diagnostic material when visceral leishmaniasis is a consideration. In aspirations, Leishmania amastigotes ingested within macrophages may be seen. Such forms are more difficult to appreciate in biopsies.
Likewise, Chagas disease (American trypanosomiasis) is very different from histoplasmosis and leishmaniasis. When Trypanosoma cruzi enters a human through contact with a triatomine bug either by fecal contamination of a mucous membrane or by biting, an inflammatory reaction with a nodular swelling or chagoma develops. The organisms are then conveyed by the lymphatics to regional lymph nodes. When the histiocytes or other inflammatory cells ingest the parasites, intracellular amastigotes are formed (Image 6). The intracellular amastigotes burst out of cells to circulate in the blood and other body fluids as trypomastigotes which are the diagnostic forms (Image 7). Endemic American trypanosomiasis is rare in the United States but has been reported in Texas, Oklahoma, Tennessee, Louisiana, and California.6
Supplementary Questions
- The urine antigen test for histoplasmosis is useful in all histoplasmosis patients.
- True
- False
- Skin testing for histoplasmin is useful for primary diagnosis of histoplasmosis.
- True
- False
- Which histochemical stain is best for the demonstration of H. capsulatum?
- GMS
- H&E
- Fontana-Mason
- Trichrome
- In an immunocompromised patient with disseminated Histoplasma capsulatum infection, which is the most sensitive and specific diagnostic test?
- Antigen detection
- Culture
- Histopathology
- Serology
References
- Wheat LJ, Azar MM, Bahr NC, et al. Histoplasmosis. Infect Dis Clin North Am. 2016;30(1):207-227.
- Benedict K, Thompson GR, Deresinski S, et al. Mycotic infections acquired outside of areas of known endemicity, United States. Emerg Infect Dis. 2015;21(11):1935-1941.
- Badami V, Farjo P, Guilfoose J. Histoplasmosis fungemia manifesting as acute respiratory distress syndrome. Am J Resp Crit Care Med. 2018;197:A5207.
- Bertolini M, Mutti MF, Barletta JAE, et al. COVID-19 associated with AIDS-related disseminated histoplasmosis: a case report. Int J STD & AIDS. 2020;31(12):1222-1224.
- Centers for Disease Control and Prevention; Division of Parasitic Disease and Malaria. Leishmaniasis. Centers for Disease Control and Prevention. Accessed October 19, 2021. https://www.cdc.gov/dpdx/leishmaniasis/index.html
- Centers for Disease Control and Prevention. Parasites - American trypanosomiasis (also known as Chagas disease). Centers for Disease Control and Prevention. Reviewed February 11, 2019. Accessed October 19, 2021. https://www.cdc.gov/parasites/chagas/index.html
- Libert D, Procop GW, Mohammad QA. Histoplasma urinary antigen testing obviates the need for coincident serum antigen testing. AJCP. 2018;149:362-368.
- Goodwin RA Jr, Owens FT, Snell JD, et al. Chronic pulmonary histoplasmosis. Medicine. 1976;55(6):413-452.
- Caceres DH, Knuth M, Derado G, Lindsley MD. Diagnosis of progressive disseminated histoplasmosis in advanced HIV: A meta-analysis of assay analytical performance. J Fungi (Basel). 2019;5(3):76. doi:10.3390/jof5030076
Authors
Gordon L. Love, MD, FCAP
Clinical Pathology Education Committee
Professor and Chair, Department of Pathology, LSU School of Medicine
Sara L. Cook, MD, PhD
Clinical Pathology Education Committee
Anatomic and Clinical Pathology Residency Program, Mayo Clinic
Answer Key
- False (b) – H. capsulatum antigen testing in urine is useful for rapid evaluation of individuals with pulmonary disease following inhalation of a large quantity of organisms or those with possible disseminated disease. False-negative reactions can be encountered in other histoplasmosis presentations. Cross-reactivity with infections of other fungi, including Blastomyces dermatitidis, may be seen. A positive H. capsulatum antigen test should be correlated with culture and other laboratory findings.7
- False (b) – Skin testing for histoplasmin has no value in patient diagnosis due to the wide-spread reactivity of people within endemic zones. The CDC estimates that between 80% to 90% of people who live in areas where the fungus is common have been exposed. Furthermore, a negative skin test is of no value in that 20% of patients with chronic pulmonary histoplasmosis have a negative skin test.8
- GMS (a) – GMS stain may be superior for staining of small yeast of Histoplasma capsulatum. Tiny yeast are highlighted with this stain.
- Antigen detection (a) – Accurate diagnosis is crucial in an immunocompromised patient with progressive disseminated disease. Among the listed diagnostic tests, antigen detection is reported to have the highest sensitivity and specificity.9 Cultures have variable sensitivity due to sample type and laboratory practices. Serology has high specificity but low sensitivity. Lastly, the sensitivity and specificity of histopathology is dependent on the experience of the pathologist.