- Home
- Laboratory Improvement
- Accreditation
- Biorepository Accreditation Program

The Biorepository Accreditation Program, established in 2012 by the College of American Pathologists (CAP)was the first accreditation program to improve the quality and consistency of biorepositories. The program aims to provide requirements for standardizing processes that will result in high-quality human specimens and genetic materials that can support research, drug discovery, and precision medicine. These requirements are part of our discipline-specific accreditation checklists which provide a clear roadmap for achieving accreditation and maintaining a high-quality biorepository.

Benefits
The CAP's biorepository accreditation demonstrates your commitment to excellence and offers several tangible benefits, including:
- Appropriate ethical and legal frameworks for the use of biospecimens in IRB-approved research
- Well-controlled pre-analytic variables for optimal scientific investigation or biomarker development
- Robust chain of custody tracking, reducing the risk of misidentification
- Strict monitoring to ensure appropriate temperatures and storage conditions
- Best practice policies and procedures for sample release
- Histologic quality assurance for starting tumor content in samples homogenized for downstream assays
- Confidence in the long-term quality of biospecimens
- Differentiation for research and grant funding opportunities
Core Attributes of Our Pioneering Program
Checklists
Drawing on the collective knowledge of our pathologists, biobanking and biopreservation experts, and best practices from the International Society for Biological and Environmental Repositories (ISBER), National Cancer Institute (NCI), Organisation for Economic Co-operation and Development (OECD), the Centers for Medicare & Medicaid Services (CMS), and our Laboratory Accreditation Program, we help your facility stay current on advancements within the industry through our checklists.
These annually updated checklists provide a clear roadmap for not only achieving accreditation but also for running a high-quality biorepository. Aligning the checklists from the Biorepository Accreditation Program (BAP) and the CAP's Laboratory Accreditation Program helps ensure:
- Confidence in specimen provenance, allowing a clinical laboratory director to accept specimens from a BAP-accredited biorepository because of formal CLIA-approved requirements in specimen collection integrity
- Confidence in pre-analytic variable tracking and control of samples used in drug trials with associated biomarker development
- Alignment of accreditation preparation and inspection processes for biorepositories affiliated with CAP-accredited laboratories
Peer Inspection Model
- Our peer inspection model is the foundation of the CAP's unique inspection program. Inspectors—Team Leaders (pathologists/PhDs, or biorepository managers) and Team Members (laboratory professionals)—must meet requirements through the CAP Inspector Training course and have current experience in a CAP-accredited biorepository/biobank.
- On-site inspections occur every two years using the CAP Accreditation Checklists to assess compliance with program requirements, with an interim self-inspection required during the off-year.
- Participants have access to the CAP's Biorepository, Laboratory General, Director Assessment, and All Common Checklists through our customer portal, e-LAB Solutions Suite. Non-accredited biorepositories can purchase the checklist.
- Following the on-site inspection, a CAP technical specialist will collaborate with the biorepository for a remote post-inspection review. This review assists biorepositories in strengthening their overall quality by identifying areas for improvement.
Educational Resources
The CAP offers complimentary educational resources to all its accredited laboratories in a customer-only portal that provides the roadmap, tools, and support needed to prepare for inspections, maintain compliance, and provide the highest level of quality.
Drawn from the collective knowledge of more than 500 laboratory experts, our extensive, frequently updated repository includes:
- Archived webinars with toolkits on topics like risk management, quality management systems, proficiency testing failures, preanalytical errors
- Laboratory Inspection Preparation Course
- CAP Laboratory Director Education, Information & Resources
- Resource toolboxes, including IQCP, PT/EQA, new laboratory director, root cause analysis
- Over 55 templates providing a starting point for demonstrating compliance, including competency, personnel, and validation & verification
- Checklist requirement Q&A’s for more than 80 common accreditation inspection deficiencies
Qualifications
A biorepository receives, stores, processes, and disseminates biospecimens, their derivatives, and relevant data for research purposes qualify. CAP accreditation covers the biorepository's physical location and all associated activities performed by biorepository personnel, including:
- Patient consent
- Specimen collection, processing, and storage
- Quality control
- Quality assurance processes, such as sample characterization testing for suitability of the sample for downstream use
- Data management
- Sample release
- Tracking
While the program is voluntary, a CAP accreditation certificate separates your biorepository from others. Only the College of American Pathologists offers a rigorous, peer-based, and constantly evolving solution for biorepository accreditation—a critical step for ensuring high-quality specimens for precision medicine, research, and drug discovery.
The Biorepository Accreditation Program is not applicable to the following:
- The BAP does not apply to tissues stored for transplant purposes; however, the CAP Laboratory Accreditation Program does inspect transplant tissue storage under the laboratory director's purview.
- Retained clinical samples not intended for research testing are not covered under the BAP.
Working toward and achieving accreditation from the CAP helped me communicate and infuse the importance of quality across all aspects of our biorepository. We've also been able to use the requirements to benchmark our progress.
Dana R. Valley, BA, ASQ CMQ/OE, CSSGB, Operations and Quality Manager, Van Andel Institute
Proficiency Testing/External Quality Assessment (PT/EQA)
Enrollment in the CAP's PT/EQA is not required under the Biorepository Accreditation Program. However, some biorepositories may choose to enroll in CAP PT/EQA as a mechanism for periodic assessment of the quality of the stored specimens. The CAP Surveys and Anatomic Pathology Education Programs may include products applicable to biorepositories.
Per ISO 20387 7.8.2.9, the biobank shall use approaches to provide objective evidence to demonstrate the comparability of biological material quality (the processing or testing output) where such methods are available and appropriate. Such approaches include proficiency testing/external quality assessment (PT/EQA) programs and approaches, including the use of:
- Certified reference materials, where available, produced by a reference material producer fulfilling the requirements of ISO 17034
- Samples previously examined
- Samples previously shared with other biobanks
- Control materials that are tested regularly in EQA programs
Assessing Readiness
A biorepository interested in the program but not confident in its readiness may purchase a set of checklists and perform a self-inspection. The CAP also has a CAP Accreditation Readiness Assessment (CARA®) that provides a high-level evaluation of the biorepository's processes using an educational approach.
Ready to Apply?
- Not sure? Learn more about your estimated costs and next steps.
- Submit a fee estimate form to receive more information about cost and next steps to become CAP accredited.
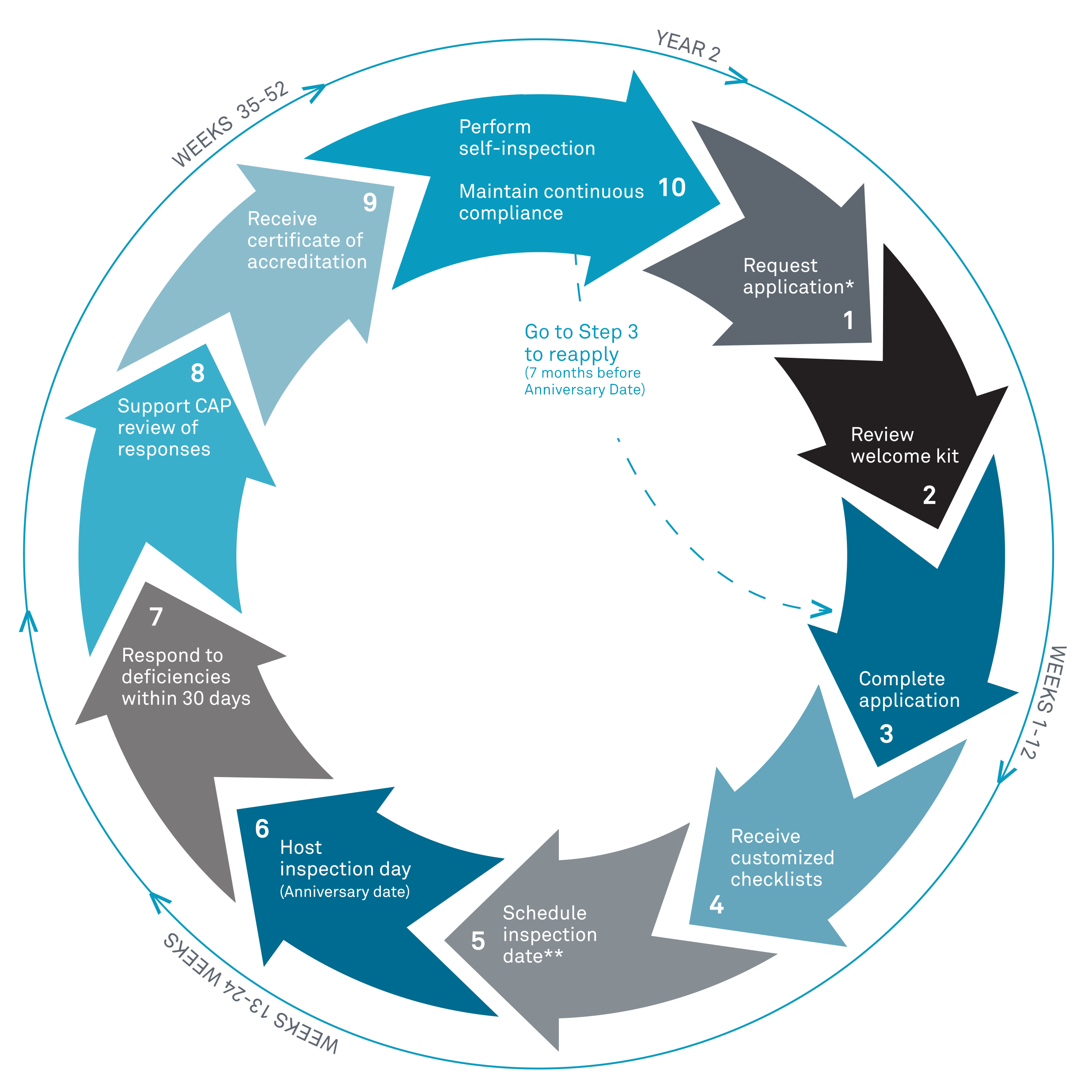
- Yes! Follow the steps below to start your biorepository journey with the CAP.
- Submit the Accreditation Request for Application form (PDF) and a one-time, non-refundable application fee.
- Once your request form and payment are processed, you'll receive an email with a link to your online Organizational Profile, which you must complete to finalize the accreditation application. You'll be asked to provide information about your biorepository, including demographic data, personnel, sections/departments and activities, and other key details.
- Once the CAP receives your finalized application (application form, payment, and completed organizational profile), CAP staff members will schedule an initial inspection readiness call with you to discuss the next steps in preparing for your inspection.

Thanks to the dedication of over 100 biorepositories worldwide, the CAP's Biorepository Accreditation Program is universally regarded as the most rigorous choice to achieve and maintain accreditation. Participation in our program demonstrates a biorepository’s commitment to the highest quality and patient care, ensuring biorepositories like yours operate with maximum confidence.
Find a CAP-Accredited Biorepository
Use our database to search for CAP-accredited biorepositories.
Hear from CAP Biorepository Experts
-
Emerging Markets and Technologies in Biobanking
Jim Vaught, PhD, Editor Emeritus of the Biopreservation and Biobanking journal, talks about the new era we are entering where new markets and technologies will result in new approaches to collecting, processing, and storing samples.
-
The Role of Biorepository Accreditation in Advancing Neurological Research
CAP's Biorepository Committee member Nalin Leelatian, MD, PhD, talks about neuro biorepositories and how the CAP's Biorepository Accreditation Program improves the quality and consistency of biobanks.
-
Why Biorepositories Have an Important Role in Supporting Medical Research
Dr. Rebecca Obeng discusses the range of services and resources that biorepositories can provide researchers as well as the benefits and value of accreditation for biorepositories.