- Home
- Member Resources
- Articles
- Cancer Immunotherapy Biomarker Testing – What Pathologists Need to Know
After reading the article, hear more background from the author on the CAPcast, Cancer Immunotherapy Biomarker Testing: What Pathologists Need to Know.
Cancer immunotherapy has revolutionized the field of oncology by delivering unprecedented levels of durable survival benefit for cancer patients, including some patients with previously incurable late-stage disease.
Anatomic and molecular pathologists have the opportunity to be at the center of the development, validation and clinical implementation of critically needed cancer immunotherapy biomarkers. Predictive immunotherapy biomarkers such as programmed death ligand-1 (PD-L1) immunohistochemistry (IHC), mismatch repair (MMR) IHC and microsatellite instability (MSI) testing are already established as routine testing in many pathology laboratories around the world, with other biomarkers and technologies still in the exploratory phase.
Clear guidelines for cancer immunotherapy testing are not currently available and the field is evolving rapidly in response to new clinical and translational data.
The intent of this webpage is to briefly summarize those cancer immunotherapy biomarkers that are in routine use as a resource for individualized test selection in clinical pathology practice.
For a more complete review of this field, please refer to the recently published article The Cancer Immunotherapy Biomarker Testing Landscape’ (Walk et. al Arch Pathol Lab Med-Vol 144, June 2020)1.
Background
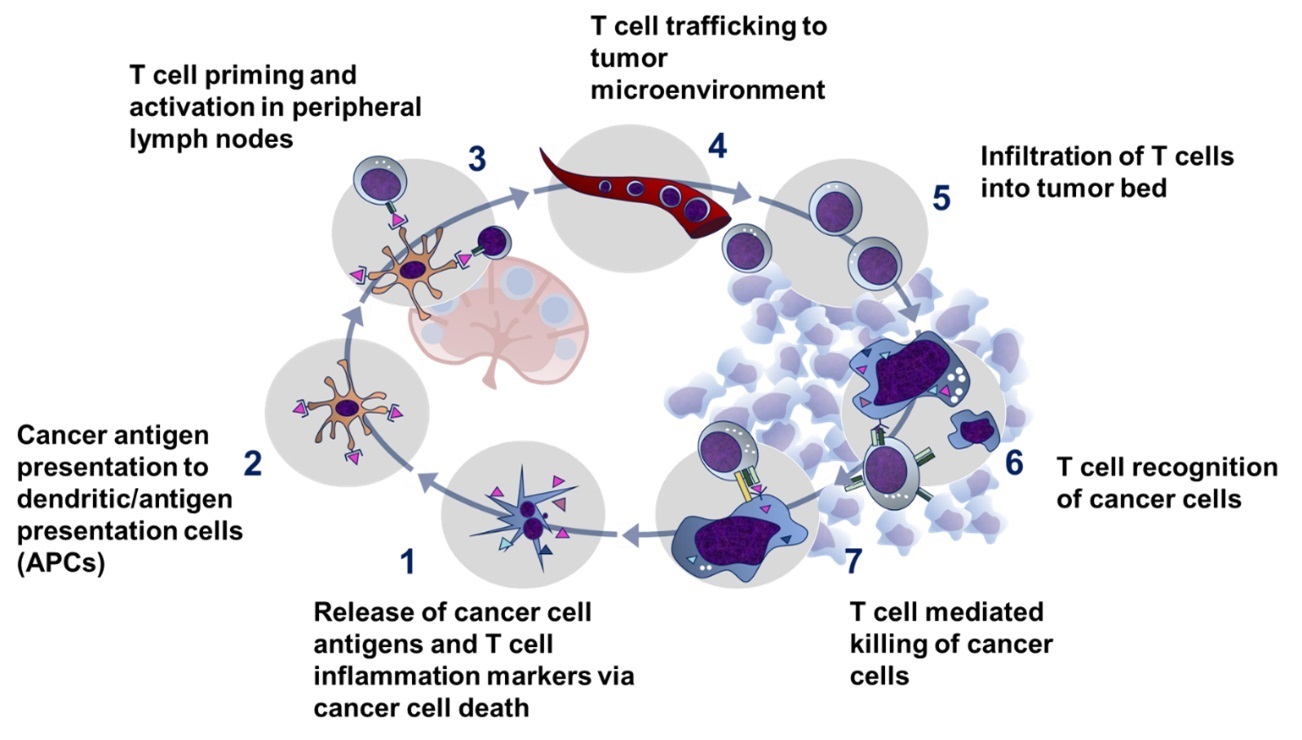
The immune-mediated elimination of cancer cells occurs via a process termed ‘the cancer immunity cycle’ (Figure 1)3. The regulation of this process is controlled via a complex system of immune checkpoint proteins on T lymphocytes and antigen-presenting cells, with some proteins activating the immune system and others down-regulating it.2

The US Food and Drug Administration (FDA) has approved multiple cancer immunotherapies, most of which target one or more immune checkpoints, over a wide range of cancer indications (download Table 1. FDA-Approved Cancer Immunotherapies [Excel])4. Over 2000 cancer immunotherapy agents are currently in clinical development.5
Since cancer immunotherapy currently only benefits about 20% of cancer patients6 and can be associated with serious adverse immune reactions7, biomarkers predicting efficacy are critically needed both for current clinical care and to drive further progress in this rapidly advancing field.
Programmed Death-Ligand 1 (PD-L1)
Five anti-PD-(L)1 therapies (atezolizumab, avelumab, durvalumab, nivolumab and pembrolizumab) have been approved by one or more global regulatory agencies across multiple cancer indications (download Table 1. FDA-Approved Cancer Immunotherapies [Excel])4.
Generally, PD-L1 expression is associated with a greater likelihood of benefit from anti-PD-(L)1 therapy, but patients with PD-L1 negative tumors also benefit from these therapies up to 20% of the time, depending on the specific study, therapy and indication.8
The FDA has accordingly designated PD-L1 as a complimentary diagnostic for some specific PD-(L)1 inhibitor-indication intended uses and as a companion diagnostic for others (download Table 2. FDA-Approved Cancer Immunotherapy Diagnostics [Excel]). A complimentary diagnostic is “a test that aids in the benefit–risk decision–making about the use of the therapeutic product, where the difference in benefit–risk is clinically meaningful,”9 in contrast to a companion diagnostic, which is an “in vitro diagnostic device or an imaging tool that provides information that is essential for the safe and effective use of a corresponding therapeutic product.”10
Four separate PD-L1 assays have been approved in association with the clinical development of the different FDA-approved anti-PD-(L)1 therapies and cancer indications, leading to distinct therapy-indication-test combinations. Download Table 2. FDA-Approved Cancer Immunotherapy Diagnostics [Excel]
This is a rapidly evolving field and changes to the current PD-L1 testing landscape are expected with approvals of additional immunotherapies, therapeutic indications and assays. Pathologists need to be aware that based on each combination of assay, therapy and indication, the PD-L1 scoring measure and threshold for positivity (i.e. cutoff) can vary. The most current version of the manufacturer’s package insert and/or the interpretation guide for each assay should be used for clinical testing and scoring.
Mismatch Repair (MMR) and Microsatellite Instability (MSI)
Testing for MMR deficiency (dMMR) and MSI has become a mandatory component of identifying patients most likely to respond to immunotherapy targeting PD-(L)1 in certain indications.
Currently, two cancer immunotherapy drugs are FDA approved for dMMR tumors based on immunohistochemistry or MSI PCR testing; pembrolizumab for treatment of unresectable or metastatic dMMR or MSI-H solid tumors that have progressed following prior treatment11, and nivolumab for dMMR or MSI-H colorectal cancer (CRC) that has progressed following therapy.12
There are two main methods of screening for MMR functional defects: IHC for the four MMR proteins MLH1, MSH2, PMS2, and MSH613 and molecular PCR (polymerase chain reaction) testing to detect MSI.14 Each method has unique limitations and diagnostic pitfalls, with IHC being dependent on fixation conditions and accurate interpretation, and MSI requiring normal tissue comparison and sometimes microdissection.15 Analytically, these methods show comparable performance with an approximately 5-10% false negative rate each.16 MMR IHC results can be falsely negative in the setting of mutations (e.g. in MLH1) that result in an antigenically intact but functionally inactive protein, while MSI can be falsely negative in the setting of intratumoral heterogeneity and inadequate microdissection.17 The choice of which method to use at a given institution usually depends on factors such as cost, availability, and turnaround time. Although using both tests will detect slightly more cases than either test alone, the benefit is small.
Tumor Mutational Burden (TMB)
Tumor mutational burden (TMB) is a measure of somatic cancer mutation prevalence typically represented as the number of mutations per megabase. Although TMB is related to dMMR and MSI, there is not complete overlap between them; most dMMR/MSI-H tumors have high TMB, but not all high TMB tumors are dMMR/MSI-H.18 For these reasons, TMB should be considered an independent parameter and should not be used interchangeably with dMMR or MSI.
Recently, the Food and Drug Administration granted accelerated approval to pembrolizumab for the treatment of adult and pediatric patients with unresectable or metastatic tumor mutational burden-high (TMB-H) [≥10 mutations/megabase (mut/Mb)] solid tumors, as determined by an FDA-approved test, that have progressed following prior treatment and who have no satisfactory alternative treatment options.19
Conclusion
As the field of cancer immunotherapy continues to develop, pathologists bear an important responsibility to lead and support studies that explore and validate novel biomarkers. The current dynamism of this field represents an opportunity for pathology to establish itself as the immunotherapy biomarker center of excellence in clinical medicine, ensuring the accurate, standardized, and timely implementation of immunotherapy diagnostics through education, training and collaboration.
Listen to the podcast
Eric Walk, MD, FCAP, Chief Medical & Scientific Officer, Senior VP of Medical & Scientific Affairs with Roche Diagnostics explains how biomarker testing has become routine, revolutionizing oncology through increased survival rates.
References
1. Walk EE, Yohe SL, Beckman A, et al. The Cancer Immunotherapy Biomarker Testing Landscape. Arch Pathol Lab Med. 2020;144(6):706-724.
2. Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12(4):252-264.
3. Chen DS, Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity. 2013;39(1):1-10.
4. U.S. Food & Drug Administration. Hematology/Oncology (Cancer) Approvals & Safety Notifications. Web site. https://www.fda.gov/drugs/informationondrugs/approveddrugs/ucm279174.htm. Accessed Nov 10th, 2020.
5. Tang J, Shalabi A, Hubbard-Lucey VM. Comprehensive analysis of the clinical immuno-oncology landscape. Ann Oncol. 2018;29(1):84-91.
6. Carretero-Gonzalez A, Lora D, Ghanem I, et al. Analysis of response rate with ANTI PD1/PD-L1 monoclonal antibodies in advanced solid tumors: a meta-analysis of randomized clinical trials. Oncotarget. 2018;9(9):8706-8715.
7. Kourie HR, Klastersky J. Immune checkpoint inhibitors side effects and management. Immunotherapy. 2016;8(7):799-807.
8. Mahoney KM, Atkins MB. Prognostic and predictive markers for the new immunotherapies. Oncology (Williston Park). 2014;28 Suppl 3:39-48.
9. Scheerens H, Malong A, Bassett K, et al. Current Status of Companion and Complementary Diagnostics: Strategic Considerations for Development and Launch. Clin Transl Sci. 2017;10(2):84-92.
10. U.S. Food & Drug Administration. List of Cleared or Approved Companion Diagnostic Devices (In Vitro and Imaging Tools). Web site. https://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/InVitroDiagnostics/ucm301431.htm. Accessed Nov 10th, 2020.
11. U.S. Food & Drug Administration. FDA grants accelerated approval to pembrolizumab for first tissue/site agnostic indication. Web site. https://www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm560040.htm. Accessed Nov 10th, 2020.
12. U.S. Food & Drug Administration. FDA grants nivolumab accelerated approval for MSI-H or dMMR colorectal cancer. Web site. https://www.fda.gov/drugs/informationondrugs/approveddrugs/ucm569366.htm. Accessed Nov 10th, 2020.
13. Arends M, Frayling I, Happerfield L, Ibrahim M. HNPCC/Lynch syndrome module: report of the immunohistochemical analysis of mismatch repair (MMR) protein expression. UK NEQAS ICC & ISH Recommendations. 2010;8.
14. Liu T, Wahlberg S, Burek E, Lindblom P, Rubio C, Lindblom A. Microsatellite instability as a predictor of a mutation in a DNA mismatch repair gene in familial colorectal cancer. Genes Chromosomes Cancer. 2000;27(1):17-25.
15. Chen W, Swanson BJ, Frankel WL. Molecular genetics of microsatellite-unstable colorectal cancer for pathologists. Diagn Pathol. 2017;12(1):24.
16. Recommendations from the EGAPP Working Group: genetic testing strategies in newly diagnosed individuals with colorectal cancer aimed at reducing morbidity and mortality from Lynch syndrome in relatives. Genet Med. 2009;11(1):35-41.
17. Muller A, Giuffre G, Edmonston TB, et al. Challenges and pitfalls in HNPCC screening by microsatellite analysis and immunohistochemistry. J Mol Diagn. 2004;6(4):308-315.
18. Vanderwalde A, Spetzler D, Xiao N, Gatalica Z, Marshall J. Microsatellite instability status determined by next-generation sequencing and compared with PD-L1 and tumor mutational burden in 11,348 patients. Cancer Med. 2018;7(3):746-756.
19. Administration USFD. FDA approves pembrolizumab for adults and children with TMB-H solid tumors. Web site. https://www.fda.gov/drugs/drug-approvals-and-databases/fda-approves-pembrolizumab-adults-and-children-tmb-h-solid-tumors. Accessed Nov 10th, 2020.

Eric Walk, MD, FCAP, is chief medical officer at PathAI and a member of the CAP Personalized Healthcare Committee. He has more than 20 years of experience in pathology, oncology drug development, precision medicine, and medical device development.