- Home
- Member Resources
- Pathology Case Challenge
- Pleural Fluid
Clinical Summary
A 54-year-old man with long-standing diabetes mellitus, chronic renal failure, and no previous history of malignancy presented with shortness of breath. His chest x-ray revealed large bilateral pleural effusions but no lung masses or adjacent lymphadenopathy.
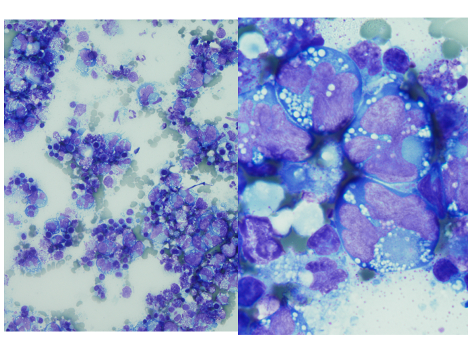
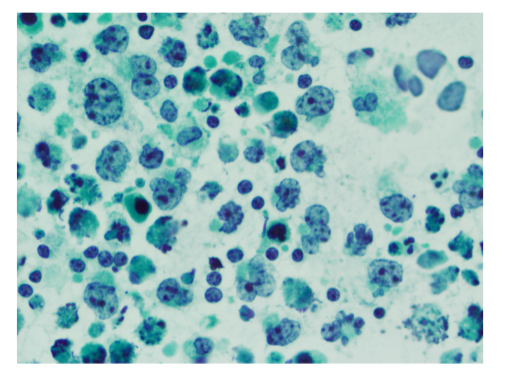
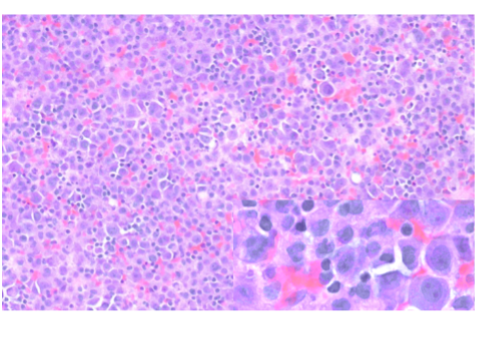
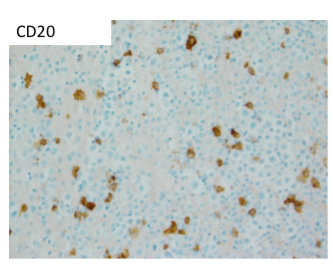
A thoracentesis was performed, and pleural fluid was sent to the hematology laboratory and cytology. The hematology technologist flagged the case for pathologist review. Images from the Wright-Giemsa-stained cytospin (Image 1), the Pap-stained smear (Image 2), and cell block (Image 3) are shown. Flow cytometric studies were reported as negative for a monotypic B-cell or aberrant T-cell population. Additional IHC studies from the cell block preparation are also shown (Images 4-7).
Master List of Diagnoses
- Metastatic carcinoma
- Primary effusion lymphoma
- Plasmablastic lymphoma
- Plasma cell myeloma
- Diffuse large B-cell lymphoma
- ALK+ large B-cell lymphoma
Archive Case and Diagnosis
This material was originally released as 2019 CPIP-L Case 12: Hematology – Dealing With Hematologic Pleural Effusions: What Not to Miss and is primary effusion lymphoma.
Criteria for Diagnosis and Comments
Morphologic review is the key starting point and guide for pathologic evaluation. Body fluids are often submitted to more than one area of the laboratory, and review of both the Wright-Giemsa-stained material from the hematology section (Image 1) and Papanicolaou (Pap)-stained material in the cytology section (Image 2) is beneficial.1,2 The high nuclear:cytoplasmic (N:C) ratios, irregular nuclear contours and nucleoli are worrisome for a malignant process, and the presence of individually dispersed tumor cells without cellular cohesion would raise the possibility of a hematolymphoid malignancy. The large cells with marked atypia would raise the possibilities of hematolymphoid malignancies such as primary effusion lymphoma (PEL), plasmablastic lymphoma (PBL), large B-cell lymphoma, and an anaplastic-appearing plasma cell myeloma. However, the differential diagnosis should also include non-hematolymphoid possibilities, such as a poorly differentiated metastatic carcinoma.
A complicating factor in establishing the diagnosis of a hematolymphoid malignancy in effusion samples is the lower sensitivity as compared to adenocarcinoma, and appropriate use of ancillary studies is crucial in the diagnostic workup.3 Flow cytometry is invaluable for the rapid characterization of the antigenic profile when a hematolymphoid malignancy is suspected; however, it requires fresh material. Fresh material can be forwarded from either of the samples originally sent to the hematology or cytology sections, with the caveat that the sample may be somewhat aged by the time it reaches the flow cytometry laboratory. Ideally flow cytometry samples should be processed within 24 hours of receipt, and delays in transit may result in selective loss of the neoplastic cells, especially with high-grade neoplasms. Alternatively, the malignant cells may not have been well represented in the sample, or the flow cytometric panel may not have been tailored for all entities in the differential diagnosis. For these reasons it is important not to be misled by the negative result in this case and discount the possibility of a hematologic malignancy without further work-up. Fortunately, cytology laboratories routinely make a cell block (Image 3) from which additional ancillary studies, including immunohistochemistry (IHC), in-situ hybridization (ISH), and certain cytogenetic and molecular assays, can be performed for additional characterization. The pathologist plays an important role in coordinating testing to ensure efficient diagnostic evaluation.
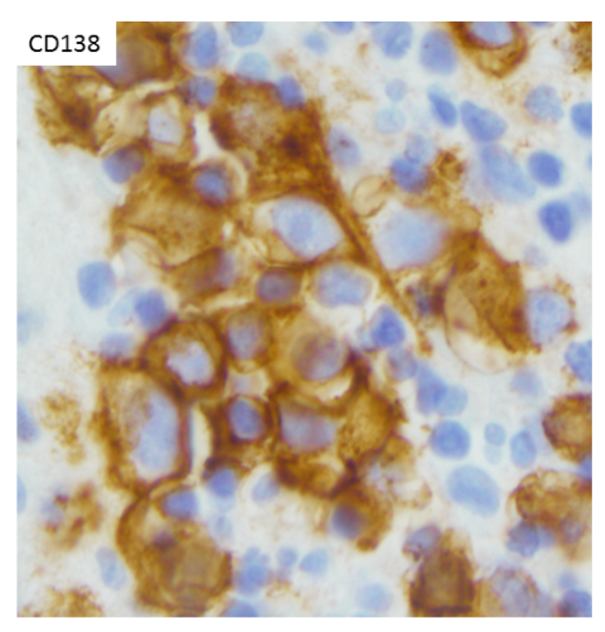
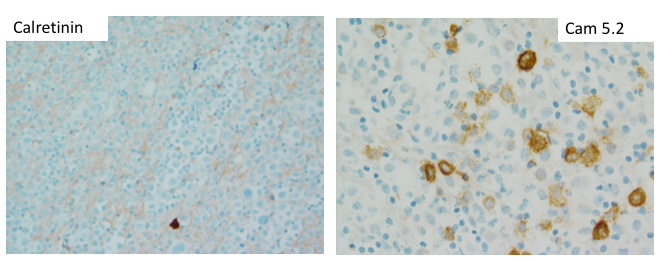
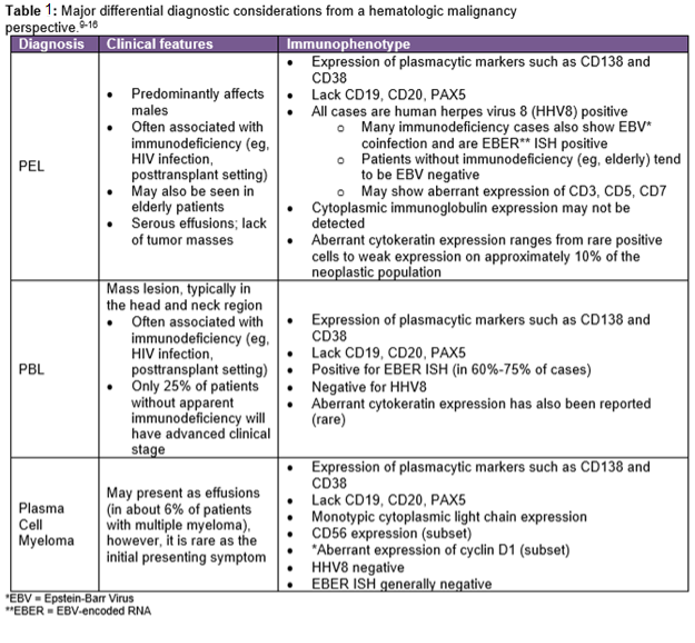
The IHC and in situ hybridization stains performed on the cell block in this case provided additional key diagnostic information (Images 4–7). These studies reveal that the neoplastic population is positive for CD138 (Image 4) and negative for CD20 (Image 5), suggestive of a B-cell neoplasm with plasmacytic/plasmablastic differentiation, although the partial positivity for the cytokeratin CAM 5.2 (Image 6) is unusual.9,17 The lack of strong CD20 expression (Image 4) would tend to exclude the most common non-Hodgkin B-cell lymphoma, diffuse large B-cell lymphoma (DLBCL).4 DLBCL associated with chronic inflammation (DLBCL-CI) typically involves body cavities as a mass in the clinical context of a long-standing pyothorax; however, DLBCL-CI is typically CD20+ and associated with EBV.9 These clinical and pathologic features are not observed in this case. ALK+ large B-cell lymphoma is a very rare subtype (<1% of large B-cell lymphomas) characterized by ALK IHC positivity and an immunoblastic/plasmablastic morphology and phenotype.9,18 Although the morphology and CD138+ CD20- phenotype overlap with this case, the clinical presentation would be unusual for ALK+ DLBCL, which more frequently presents as generalized lymphadenopathy or a mass lesion in younger patients (median age of 43 years).19 For these reasons, the main differential diagnostic considerations are PEL, PBL, and plasma cell myeloma, all of which may show a morphological spectrum from sheets of large, pleomorphic, immunoblastic, or frankly anaplastic malignant cells, to cells with more obvious plasmacytic differentiation. Table 1 summarizes the clinicopathologic features of PEL, PBL, and plasma cell myeloma.
The CD138 cell surface antigen (syndecan-1) is a specific marker of plasmacytic differentiation for hematolymphoid neoplasms, however, is also present on the surface of epithelial cells due to its role in cellular adhesion. The neoplastic population lacks expression of the mesothelial marker calretinin, however, does show some expression of the epithelial marker CAM5.2 (Image 6). Therefore, the CD138 expression (Image 5), together with the partial CAM 5.2 expression (Image 6), may also cause concern for involvement by an epithelial neoplasm. Interestingly, all three entities in the main differential diagnosis, PEL, PBL, and plasma cell myeloma, may show some type of positivity for epithelial markers.5,6 The most common aberrant epithelial marker expressed is EMA,5 other epithelial markers that may be expressed aberrantly in plasmacytic or plasmablastic B-cell neoplasms include AE1/AE3, CK22, and CAM 5.2.6-8 A clue may be the pattern of expression; when epithelial markers are aberrantly present on an otherwise characteristic hematolymphoid neoplasm, the expression tends to be weak and on a subset of cells, as seen with the CAM 5.2 positivity in this case (Image 6).
Plasma cell myeloma uncommonly presents initially as a malignant pleural effusion, however, given the clinical history of renal failure, this entity remains in the “top 3” differential diagnostic considerations. Clinical correlation, including bone marrow evaluation and evaluation for a monoclonal serum or urine paraprotein, is especially helpful when plasma cell myeloma is a diagnostic consideration, as there is often disease elsewhere. Cyclin D1 is a useful additional marker, as positivity would strongly favor plasma cell myeloma, since it is not present in either PBL or PEL, and the other types of cyclin D1+ mature B-cell lymphoid neoplasms (mantle cell lymphoma, hairy cell leukemia, DLBCL) typically lack a plasmacytic/plasmablastic immunoprofile. The phenotype of plasma cell myeloma (CD138+, CD20-, cytoplasmic light chain restricted) overlaps with PBL, however, plasma cell myeloma generally does not show the EBV positivity characteristic of PBL (seen in 60%-75% of PBL cases).9
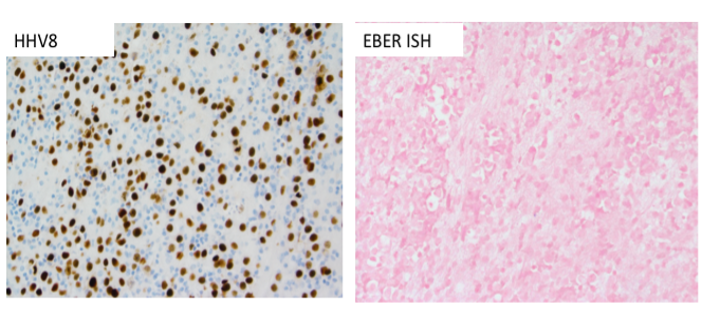
In this case, the HHV8 positivity (Image 7) is a key diagnostic feature, as it is universally associated with PEL. Most PEL cases will show co-expression of HHV8 with EBV; even though this case is EBER ISH negative, this would not preclude establishing the diagnosis of PEL, as a subset of PEL will lack EBV positivity.9 The HHV8 positivity would exclude other entities in the differential diagnosis (plasma cell myeloma and PBL), and together with the overall morphologic and immunophenotypic features, would establish the diagnosis of PEL.
Most cases of PEL arise in young or middle-aged male patients with a history of HIV infection and immunodeficiency. However, given that EBV-negative PEL typically occurs in older patients from HHV8-endemic areas such as the Mediterranean, this patient may not have a history of acquired immunodeficiency/HIV.9,20 Although most cases of PEL remain restricted to the body cavities, some show dissemination and/or extracavitary tumors, and the prognosis is generally poor.
This finding of a new malignancy may not be expected and should be discussed with the clinical team. During this discussion, it would be helpful to ascertain if the patient was immunocompromised in any way (eg, HIV infection, posttransplant) or if he was from an HHV8-endemic area. The need for an immediate referral to hematology/oncology should also be communicated. Despite the poor prognosis, recent studies suggest that high-dose therapy and autologous hematopoietic stem cell transplantation may be effective for this rare tumor, although the high relapse rate is a limiting factor.21
Supplementary Questions
- Which of the following results would be most helpful in establishing a diagnosis of primary effusion lymphoma?
- CD20+
- EBV+
- HHV8+
- CD138+
- True or False: Cyclin D1 positivity is typically associated with plasmablastic lymphoma.
- True
- False
References
- Galagan KA, Bloomberg D, Cornbleet PJ, et al, eds. Color Atlas of Body Fluids. CAP; 2006.
- Rabinovitch A, Cornbleet PJ. Body fluid microscopy in US laboratories. Data from two College of American Pathologists surveys, with practice recommendations. Arch Pathol Lab Med. 1994;118(1):13-17.
- Arnold DT, De Fonseka D, Perry S, et al. Investigating unilateral pleural effusions: the role of cytology. Eur Respir J. 2018;52(5).
- Vega F, Padula A, Valbuena JR, et al. Lymphomas involving the pleura: a clinicopathologic study of 34 cases diagnosed by pleural biopsy. Arch Pathol Lab Med. 2006;130(10):1497-1502.
- Baldus SE, Palmen C, Thiele J. MUC1 (EMA) expressing plasma cells in bone marrow infiltrated by plasma cell myeloma. Histol Histopathol. 2007;22(8):889-893.
- Adams H, Schmid P, Dirnhofer S, Tzankov A. Cytokeratin expression in hematological neoplasms: a tissue microarray study on 866 lymphoma and leukemia cases. Pathol Res Pract. 2008;204(8):569-573.
- Bharani V, Sharma P, Bal A, Prakash G. A plasma cell myeloma with post-therapy anaplastic morphology, osteomyelosclerosis, and strong pan-cytokeratin (AE1/AE3) expression: A potential diagnostic pitfall. Int J Surg Pathol. 2018;26(3):232-235.
- Xu X, Roberts SA, Pasha TL, et al. Undesirable cytokeratin immunoreactivity of native nonepithelial cells in sentinel lymph nodes from patients with breast carcinoma. Arch Pathol Lab Med. 2000;124(9):1310-1313.
- Swerdlow S, Campo E, Harris NL, et al, eds. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. IARC; 2017.
- O'Connell FP, Pinkus JL, Pinkus GS. CD138 (syndecan-1), a plasma cell marker IHC profile in hematopoietic and nonhematopoietic neoplasms. Am J Clin Pathol. 2004;121(2):254-263.
- Courville EL, Sohani AR, Hasserjian RP, et al. Diverse clinicopathologic features in human herpesvirus 8-associated lymphomas lead to diagnostic problems. Am J Clin Pathol. 2014;142(6):816-829.
- Yamani F, Chen W. Cytokeratin-positive primary effusion lymphoma: a diagnostic challenge. Br J Haematol. 2018;180(1):9.
- Wang HY, Wang L. Diagnostic pitfall: primary effusion lymphoma with rare cytokeratin immunoreactivity. Blood. 2016;127(24):3102.
- Castillo JJ, Bibas M, Miranda RN. The biology and treatment of plasmablastic lymphoma. Blood. 2015;125(15):2323-2330.
- Kim JE, Kim YA, Kim WY, et al. Human immunodeficiency virus-negative plasmablastic lymphoma in Korea. Leuk Lymphoma. 2009;50(4):582-587.
- Chen H, Li P, Xie Y, et al. Cytology and clinical features of myelomatous pleural effusion: Three case reports and a review of the literature. Diagn Cytopathol. 2018;46(7):604-609.
- Galán J, Martin I, Carmona I, et al. The utility of multiparametric flow cytometry in the detection of primary effusion lymphoma (PEL). Cytometry B Clin Cytom. 2019;96(5):375-378.
- Reichard KK, McKenna RW, Kroft SH. ALK-positive diffuse large B-cell lymphoma: report of four cases and review of the literature. Mod Pathol. 2007;20(3):310-319.
- Laurent C, Do C, Gascoyne RD, et al. Anaplastic lymphoma kinase-positive diffuse large B-cell lymphoma: a rare clinicopathologic entity with poor prognosis. J Clin Oncol. 2009;27(25):4211-4216.
- Song JY, Jaffe ES. HHV-8-positive but EBV-negative primary effusion lymphoma. Blood. 2013;122(23):3712.
- Mirza AS, Dholaria BR, Hussaini M, et al. High-dose therapy and autologous hematopoietic cell transplantation as consolidation treatment for primary effusion lymphoma. Clin Lymphoma Myeloma Leuk. 2019;19(9):e513-e520. doi:10.1016/j.clml2019.03.021
Author
Excerpted for Case of the Month by:
Christine G. Roth, MD, MMM, FCAP
Clinical Pathology Education Committee
Baylor College of Medicine
Houston, TX
Answer Key
- HHV8 (c)
HHV8 is universally associated with PEL. PEL typically shows coinfection with EBV in many—but not all—cases, especially in older patients from HHV8-endemic areas. CD138 would indicate plasmacytic/plasmablastic differentiation, however, is not specific for primary effusion lymphoma. Expression of the B lymphoid marker CD20 is not typical of PEL. - False (b)
Plasmablastic lymphoma is typically negative for cyclin D1. Cyclin D1 positivity may be seen with plasma cell myeloma, as well as mantle cell lymphoma and other hematolymphoid neoplasms.