- Home
- Member Resources
- Pathology Case Challenge
- Hematology
Clinical Summary
A 62-year-old engineer presented with fatigue, pallor, and weight loss for the past two months. Clinical examination revealed a palpable spleen and skin pallor. No lymphadenopathy was detected clinically. Ultrasound examination confirmed the presence of an enlarged spleen (20 cm) and mild hepatomegaly. Initial laboratory workup included a CBC and differential with values shown in Table 1. Given these findings, a bone marrow aspirate and biopsy were subsequently performed.
The bone marrow aspirate differential count showed a blast population comprising 39% of total cells counted. Flow cytometry showed a myeloblast population that was positive for CD34, HLA-DR, CD33, CD13, and CD117. Microscopic bone marrow aspirate findings are shown in Image 1.
Master List of potential etiologic agents:
- Essential thrombocythemia (ET)
- Primary myelofibrosis (PMF)
- Chronic myeloid leukemia (CML) blast phase
- Acute myeloid leukemia (AML) with inv(3)
Table 1: CBC and differential.
Analyte |
Result |
Units of Measure |
Reference Range |
Hemoglobin (Hg) |
5.5L |
g/dL |
11.8-14.7 |
Hematocrit (Hct) |
17.6L |
% |
35.0-43.0 |
Red blood cells (RBC) |
1.96L |
x 1012/L |
4.2-5.3 |
Mean corpuscular volume (MCV) |
96.3H |
fL |
77-91 |
White blood cells (WBC) |
8.9 |
x 109/L |
3.8-10.4 |
Absolute neutrophil count |
4.9 |
x 109/L |
1.8-7.2 |
Absolute lymphocyte count |
2.4 |
x 109/L |
1.0-3.2 |
Absolute monocyte count |
0.4 |
x 109/L |
0.2-0.8 |
Absolute eosinophil count |
0.0 |
x 109/L |
0.1-0.2 |
Absolute basophil count |
0 |
x 109/L |
0.00-0.01 |
Blasts |
23H |
% |
0 |
Bands |
12.1 |
% |
0 |
Platelet count (Plt) |
495H |
x 109/L |
187-400 |
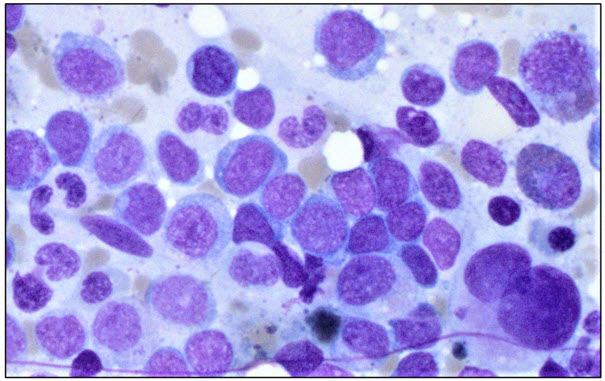
Image 1: Bone marrow aspirate. Bone marrow aspirate field showing hypolobate
megakaryocytes and increase in blasts.
Criteria for Diagnosis and Comments
This material was originally released as 2022 CPIP-G Case 7: Hematology – Thrombocytosis: An Integrated Diagnostic Approach.
Thrombocytosis is defined as a platelet count >450 x 109/L. One of the most important steps in the workup for thrombocytosis is to determine whether the cause is a primary bone marrow disorder or a secondary reactive process. This can often be accomplished through a combination of patient history, physical exam findings, and interpretation of the CBC within the context of additional laboratory findings. If a primary cause is suspected, bone marrow evaluation is recommended.
Thrombocytosis is a frequently encountered finding in both myeloproliferative and myelodysplastic syndromes and with certain types of acute myeloid leukemia (AML). A combination of morphologic, phenotypic, and molecular findings can help determine the diagnosis.
The case described above included a high platelet count with dysplastic megakaryocytes seen on morphologic evaluation of the bone marrow. These findings are relatively nonspecific on their own, however the presence of >20% myeloblasts in this case greatly helps to narrow down the diagnosis to an AML associated with thrombocytosis and megakaryocyte dysplasia. The types of that can present with these features include AML with inv(3) and AML arising in association with myelodysplastic syndrome. Cytogenetics and FISH studies were positive for inv(3)(q21.3q26.2) mutation confirming the diagnosis of AML with inv(3), which is referred to as AML with MECOM rearrangement in the most recent World Health Organization (WHO) edition. This mutation results in juxtaposition of the GATA2 enhancer to the EVI1 gene leading to overexpression of EVI1 and functional loss of GATA2, a critical component of normal hematopoiesis.
Features required for the diagnosis of this entity include increased blast count, though sometimes blasts may be <20%. There must be no history of myeloproliferative neoplasm and no fulfillment of criteria for myeloid neoplasm post-cytotoxic therapy. Detection of MECOM rearrangement must be confirmed by cytogenetic analysis, FISH, or molecular studies. While most cases involve the inv(3) mutation resulting in GATA2::MECOM rearrangement, there are cases involving MECOM rearrangements with other gene partners. The disease course is aggressive with survival of seven to 10 months. Complex karyotype with additional monosomy 7 and the presence of two or more additional mutations is associated with even worse prognosis.
Supplementary Questions
- Which of the following findings would be most useful for differentiating AML with inv (3)(q21.3q26.2) from myeloproliferative neoplasms (MPN)?
- Thrombocytosis
- Megakaryocyte dysplasia
- Blast count >20%
- Marrow fibrosis
- AML with inv (3)(q21.3q26.2) carries a poor prognosis.
- True
- False
Authors
Excerpted for Case of the Month by:
Robert Bubar, MD, Junior Member
Vandita P. Johari, MD, FCAP, Chair
Clinical Pathology Education Committee
References
- Alaggio R, Amador C, Anagnostopoulos I, et al. The 5th edition of the World Health Organization Classification of Haematolymphoid Tumours: Lymphoid Neoplasms. Leukemia. 2022;36(7):1720-1748.
- Thakral B, Wang SA. Thrombocytosis. In: Wang SA, Hasserjian RP, eds. Diagnosis of Blood and Bone Marrow Disorders. Springer International; 2018:225-242.
- Tefferi A, Hanson CA, Inwards DJ. How to interpret and pursue an abnormal complete blood cell count in adults. Mayo Clin Proc. 2005;80(7):923-936.
- Kim SY, Kyongok I, Park SN, et al. CALR, JAK2, and MPL mutation profiles in patients with four different subtypes of myeloproliferative neoplasms: primary myelofibrosis, essential thrombocythemia, polycythemia vera, and myeloproliferative neoplasm, unclassifiable. Am J Clin Pathol. 2015;143(5):635-644.
- Barbui T, Thiele J, Gisslinger H, et al. The 2016 WHO classification and diagnostic criteria for myeloproliferative neoplasms: document summary and in-depth discussion. Blood Cancer J. 2018;8(2):15.
- Barbui T, Thiele J, Vannucchi AM, et al. Rationale for revision and proposed changes of the WHO diagnostic criteria for polycythemia vera, essential thrombocythemia and primary myelofibrosis. Blood Cancer J. 2015;5(8):e337.
- Swerdlow SH, Campo E, Harris NL, et al, eds. WHO Classification of Tumours of the Hematopoietic and Lymphoid Tissues. 4th ed. IARC; 2017.
- Rumi E, Cazzola M. How I treat essential thrombocythemia. Blood. 2022;128(20):2403-2414.
- Rumi E, Cazzola M. Diagnosis, risk stratification, and response evaluation in classical myeloproliferative neoplasms. Blood. 2017;129(6):680-692.
- Hasserjan RP, Head DR. Myelodysplastic syndromes. In: Jaffe ES, Arber DA, Campo E, Harris NL, Quintanilla L, eds. Hematopathology. Elsevier; 2017:793-816.
- Mallo M, Cervera J, Schanz J, et al. Impact of adjunct cytogenetic abnormalities for prognostic stratification in patients with myelodysplastic syndrome and deletion 5q. Leukemia. 2011;25(1):110-120.
- Patnaik MM, Lasho TL, Finke CM, et al. WHO-defined 'myelodysplastic syndrome with isolated del(5q)' in 88 consecutive patients: survival data, leukemic transformation rates and prevalence of JAK2, MPL and IDH mutations. Leukemia. 2010;24(7):1283-1289.
- List A, Dewald G, Bennett J, et al. Lenalidomide in the myelodysplastic syndrome with chromosome 5q deletion. N Engl J Med. 2006;355:1456-1465.
- Zahr AA, Salama ME, Carreau N, et al. Bone marrow fibrosis in myelofibrosis: pathogenesis, prognosis and targeted strategies. Haematologica. 2016;101(6):660-671.
Answer Key
- Blast count >20% (c) - Thrombocytosis can be seen in essential thrombocythemia (ET) while megakaryocyte dysplasia and marrow fibrosis can be seen in primary myelofibrosis (PMF). A blast count >20% suggests that one is dealing with a form of AML.
- True (a) - The presence of inv(3) is associated with poor response to therapy, early relapse, and poor life expectancy of only seven to 10 months compared to other subtypes of AML.