- Home
- Member Resources
- Pathology Case Challenge
- Inguinal Soft Tissue
Clinical Summary

A 58-year-old man presents with a 5-year history of a painless, slow-growing subcutaneous mass in the inguinal region. Physical examination of the area identifies a firm, mobile mass that is 15 cm in greatest dimension. He undergoes surgical resection. Immunohistochemical stains show that the lesional cells are positive for CD34 and STAT6 and negative for keratin, EMA, S100 protein, SMA, MDM2, and h-caldesmon.
Master List of Diagnoses:
- Dedifferentiated liposarcoma
- Dermatofibrosarcoma protuberans
- Glomus tumor
- Solitary fibrous tumor
- Synovial sarcoma, monophasic
Archive Case and Diagnosis
This case first appeared as Performance Improvement Program in Surgical Pathology (PIP) 2021, Case 02, and is solitary fibrous tumor of the inguinal soft tissue.
The information provided in this case was accurate and correct at the time of publication in 2021.
Any changes in terminology since the time of publication may not be reflected in this case.
Criteria for Diagnosis and Comments
Microscopic review of the tumor shows a relatively well-circumscribed hypercellular spindle cell neoplasm with scattered ectatic vessels and focal increased mitotic activity. The overall findings are consistent with a solitary fibrous tumor (SFT).
SFT is a fibroblastic mesenchymal neoplasm of intermediate (rarely metastasizing) biological potential. SFT is primarily a tumor of adults (20 - 70 years) and affects both sexes equally. SFT was originally described as a neoplasm of the pleura and mediastinum. It is now known to arise virtually anywhere, including soft tissue and visceral locations (eg, abdomen/pelvis, extremity, trunk, head/neck, meninges). Microscopically, SFTs are composed of uniform spindled to ovoid cells with varying degrees of cellularity. The cells are often randomly arranged in a “patternless” pattern with fibrous stroma, densely collagenized foci, and prominent staghorn vasculature (ectatic vessels with irregular, antler-like shapes). Necrosis is usually absent, and the mitotic activity is frequently low (fewer than 3 per 10 high-power fields [HPF]). SFTs may be regarded as “great imitators” in soft tissue lesions, as they have a wide morphologic spectrum. Cellularity alone varies greatly, and tumors once designated as hemangiopericytoma are now considered to be a cellular variant of SFT. Other unusual variants include SFT with prominent myxoid change, mature fat (lipomatous variant), and multinucleated tumor giant cells (also known as giant cell angiofibroma).
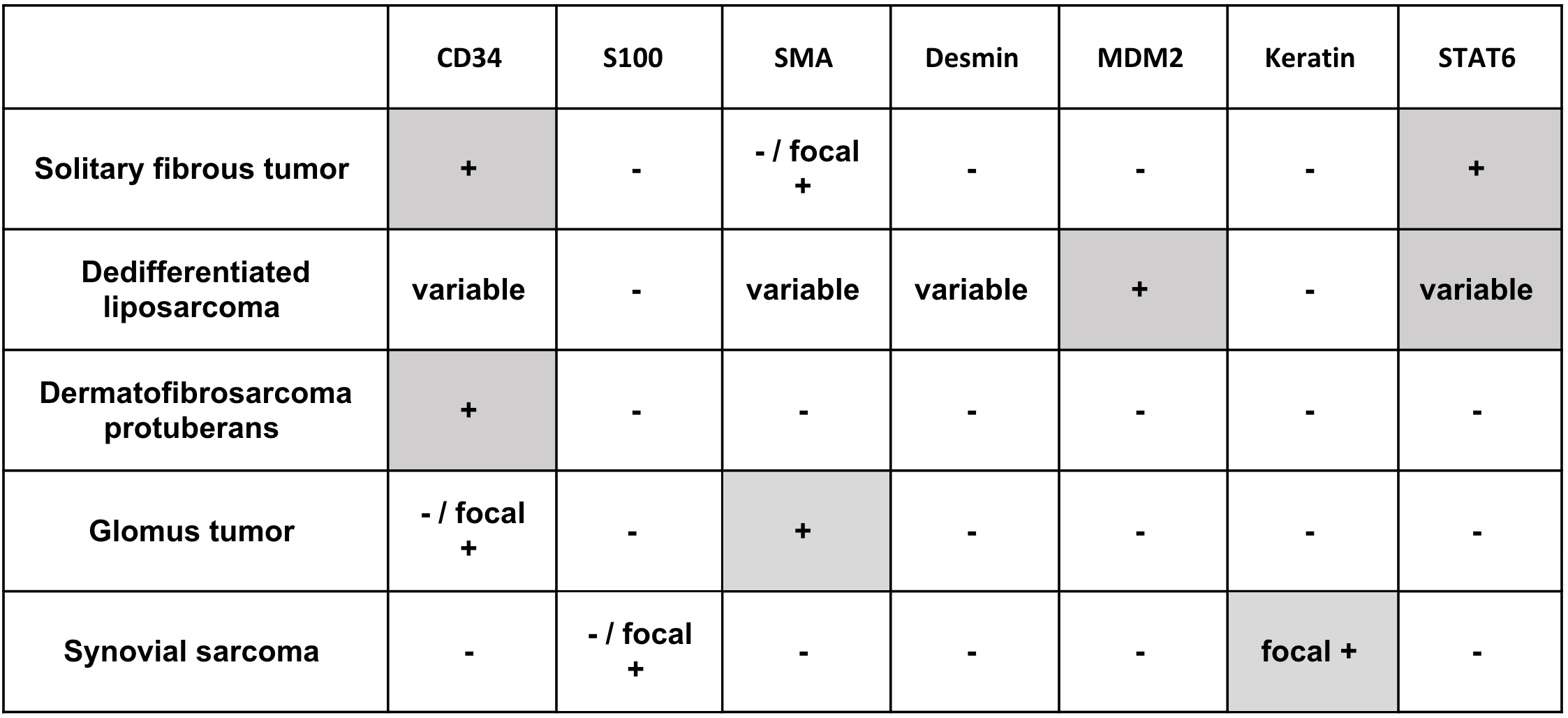
SFT are usually well-circumscribed but unencapsulated lesions with a homogeneous, firm, gray-white cut surface. Immunohistochemical stains show that the tumor cells are positive for CD34 (strong, diffuse), STAT6 (strong, nuclear), vimentin, CD99, and BCL2, while they are typically negative for keratin, EMA, S100 protein, SMA, and h-caldesmon. Molecular analysis shows a characteristic NAB2::STAT6 fusion, which induces expression of BCL2, thereby promoting cellular survival. The fusion results from a paracentric inversion affecting the long arm of chromosome 12, which is too small to reliably detect by fluorescence in situ hybridization (FISH). Accordingly, STAT6 expression by immunohistochemistry is considered a recent and reliable finding in SFT.
Most SFT are benign (85% - 90%) and typically present as a slow-growing, painless mass. A small subset of patients presents with symptoms related to paraneoplastic hypoglycemia due to the production of insulin-like growth factors I and II by the tumor. While most are indolent, some tumors are aggressive, with multiple recurrences and distant metastases (often involving lung, liver, and bone). Unfortunately, there is no precise correlation between tumor morphology and behavior. While most SFTs with aggressive behavior show overtly malignant features, occasional benign-appearing SFTs can give rise to metastases. Proposed criteria for malignancy include the following: large size, disseminated disease at presentation, increased mitotic activity (greater than or equal to 4 mitotic figures per 10 HPF), high cellularity, nuclear pleomorphism, tumor necrosis, and infiltrative margins. In 2012, Demicco et al proposed a risk stratification model for SFTs incorporating patient age at presentation (greater than or equal to 55 years), tumor size (multiple cutoffs), and mitotic index (multiple cutoffs) to determine aggressive tumors with a higher risk of metastasis. Several recent studies have also suggested that molecular factors (eg, TP53 mutations, TERT promoter mutations, APAF1 mutations, and specific variants of NAB2::STAT6 gene fusions) may be related to malignant behavior. Rare cases of SFT have additionally been reported to show an abrupt transition from typical areas to a high-grade pleomorphic sarcoma, which is thought to represent a “dedifferentiated” SFT. Most cases with reported dedifferentiation have had a particularly poor prognosis with significant metastatic disease. Due to the unpredictable behavior of these tumors, no SFT should be regarded as completely benign, and long-term clinical follow-up following surgical resection with negative margins is highly recommended.
SFT has a wide morphologic spectrum, and the differential diagnosis contains both benign and malignant entities. The lipomatous variant of SFT can be mistaken for well-differentiated or dedifferentiated liposarcoma. Rare cases of dedifferentiated liposarcoma can have SFT-like morphology, and a subset of both well-differentiated and dedifferentiated liposarcomas are positive for STAT6. The degree of STAT6 expression in SFT is usually more diffuse and stronger in intensity than dedifferentiated liposarcoma. In contrast to the strong nuclear localization of STAT6 in SFT, weaker cytoplasmic and nuclear expression are seen in dedifferentiated liposarcoma. Also, in contrast to SFT, dedifferentiated liposarcomas are positive for MDM2 and CDK4 by immunohistochemistry and demonstrate MDM2 gene amplification by FISH. Dedifferentiated liposarcomas have infiltrative borders and often have an adjacent well-differentiated component.
While dermatofibrosarcoma protuberans (DFSP) is positive for CD34, it is negative for STAT6 and lacks the characteristic staghorn vascular pattern of SFT. DFSP tends to be more superficial than SFT, with a prominent dermal component. Instead of the “patternless” pattern of SFT, it usually has a well-developed storiform pattern, as well as a characteristic chromosomal translocation t(17;22) with COL1A1::PDGFB fusion.
Glomus tumors are perivascular mesenchymal neoplasms that frequently present as a solitary lesion in the distal extremity. The tumor cells resemble modified smooth muscle cells. They are positive for smooth muscle actin and caldesmon and show abundant pericellular collagen type IV. Unlike SFT, they are predominantly negative for CD34 and STAT6. While most glomus tumors are benign, rare malignant tumors include those with marked nuclear atypia and any mitotic activity, as well as those with atypical mitotic figures. NOTCH2 rearrangements have been associated with malignant glomus tumors; BRAF V600E, and KRAS mutations have also been reported in glomus tumors.
Monophasic synovial sarcoma is a hypercellular spindle cell sarcoma that often demonstrates fascicular growth. Some poorly differentiated synovial sarcomas can have a prominent staghorn vasculature pattern mimicking SFT. Unlike SFT, CD34, and STAT6 expression are not seen in synovial sarcoma. In contrast, synovial sarcomas often have strong, diffuse nuclear expression of TLE1, as well as focal cytokeratin and EMA expression. They also have a characteristic translocation t(X;18) resulting in SS18::SSX1/2 fusions. In addition, SFTs usually lack the fascicular (herringbone or fibrosarcoma-like) architecture and uniform nuclear morphology of synovial sarcoma.
Immunohistochemistry:
Supplementary Questions
- Which of the following tumors is positive for CD34 and has an intrachromosomal rearrangement resulting in NAB2::STAT6 fusion?
- Dedifferentiated liposarcoma
- Dermatofibrosarcoma protuberans
- Glomus tumor
- Solitary fibrous tumor
- Synovial sarcoma
- A small subset of patients with solitary fibrous tumor present with which of the following symptoms/issues?
- Flushing and diarrhea from production of serotonin by the tumor
- Hypercalcemia due to secretion of parathyroid hormone-related protein by the tumor
- Hypoglycemia due to production of insulin-like growth factors by the tumor
- Muscle weakness from the production of antibodies against presynaptic voltage-gated calcium channels
- Osteomalacia from oversecretion of fibroblastic growth factor-23 by the tumor
- Which of the following molecular alterations is commonly observed in dedifferentiated liposarcoma?
- MDM2 gene amplification
- NOTCH2 rearrangements
- TERT promoter mutations
- Translocation t(X;18) resulting in SS18::SSX1/2 fusions
- Translocation t(17;22) with COL1A1::PDGFB fusion
References
- Barthelmess S, Geddert H, Boltze C, et al. Solitary fibrous tumors/hemangiopericytomas with different variants of the NAB2-STAT6 gene fusion are characterized by specific histomorphology and distinct clinicopathologic features. Am J Pathol. 2014;184:1209-1218.
- Chakrapani A, Warrick A, Nelson D, et al. BRAF and KRAS mutations in sporadic glomus tumors. Am J Dermatopathol. 2012;34(5):533-535.
- Chuang IC, Liao KC, Huang HY, et al. NAB2-STAT6 gene fusion and STAT6 immunoexpression in extrathoracic solitary fibrous tumors: the association between fusion variants and locations. Pathol Int. 2016;66(5):288-296.
- Demicco EG, Park MS, Araujo DM, et al. Solitary fibrous tumor: a clinicopathological study of 110 cases and proposed risk assessment model. Mod Pathol. 2012;25(9):1298-1306.
- Doyle LA, Tao D, Mariño-Enríquez A. STAT6 is amplified in a subset of dedifferentiated liposarcoma. Mod Pathol. 2014;27(9):1231-1237.
- Enzinger FM, Smith BH. Hemangiopericytoma. An analysis of 106 cases. Hum Pathol. 1976;7(1):61-82.
- Folpe AL. “Hey! Whatever happened to hemangiopericytoma and fibrosarcoma?” An update on selected conceptual advances in soft tissue pathology which have occurred over the past 50 years. Hum Pathol. 2020;95:113-136.
- Folpe AL, Fanburg-Smith JC, Miettinen M, et al. Atypical and malignant glomus tumors: analysis of 53 cases, with a proposal for the reclassification of glomus tumors. Am J Surg Pathol. 2001;25(1):1-12.
- Gold JS, Antonescu CR, Hajdu C, et al. Clinicopathologic correlates of solitary fibrous tumors. Cancer. 2002;94(4):1057-1068.
- Hasegawa T, Matsuno Y, Shimoda T, et al. Extrathoracic solitary fibrous tumors: their histological variability and potentially aggressive behavior. Hum Pathol. 1999;30(12):1464-1473.
- Hornick JL. Practical Soft Tissue Pathology: A Diagnostic Approach. 2nd ed. Elsevier; 2019;40-46.
- Lindberg MR. Diagnostic Pathology Soft Tissue Tumors. 2nd ed. Elsevier; 2016:176-183.
- Machado I, Nieto-Morales G, Cruz J, et al. Controversial issues in soft tissue solitary fibrous tumors: A pathological and molecular review. Pathol Int. 2020;70(3):129-139.
- Miettinen M. Modern Soft Tissue Pathology: Tumors and Non-Neoplastic Conditions. 2nd ed. Cambridge University Press; 2016;324-335.
- Mosquera JM, Sboner A, Zhang L, et al. Novel MIR143-NOTCH fusions in benign and malignant glomus tumors. Genes Chromosomes Cancer. 2013;52(11):1075-1087.
- Tai HC, Chuang IC, Chen TC, et al. NAB2-STAT6 fusion types account for clinicopathological variations in solitary fibrous tumors. Mod Pathol. 2015;28(10):1324-1335.
Answer Key
- Solitary fibrous tumor (d)
- Hypoglycemia due to production of insulin-like growth factors by the tumor (c)
- MDM2 gene amplification (a)